

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50121025 (E)-1-[2,4-Dihydroxy-5-(3-methyl-but-2-enyl)-phenyl]-3-(3,4-dihydroxy-phenyl)-propenone::CHEMBL115452::broussochalcone::broussochalcone A::med.21724, Compound 177
SMILES: [#6]\[#6](-[#6])=[#6]\[#6]-c1cc(-[#6](=O)\[#6]=[#6]\c2ccc(-[#8])c(-[#8])c2)c(-[#8])cc1-[#8]
InChI Key: InChIKey=FEALTYYKRMRXTG-QPJJXVBHSA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha glucosidase (Homo sapiens (Human)) | BDBM50121025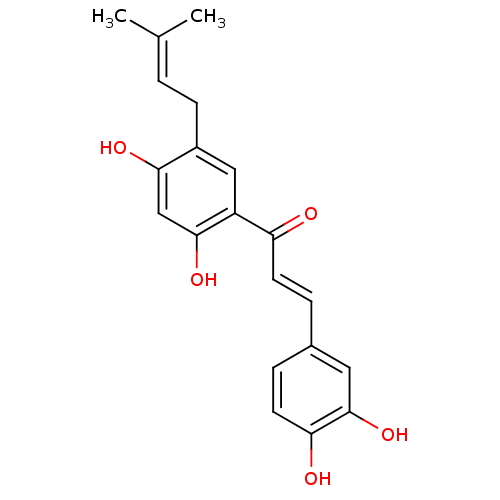 ((E)-1-[2,4-Dihydroxy-5-(3-methyl-but-2-enyl)-pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine phosphatase 1B (Homo sapiens (Human)) | BDBM50121025 ((E)-1-[2,4-Dihydroxy-5-(3-methyl-but-2-enyl)-pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Ghasidas Vishwavidyalaya (A Central University) Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) | Eur J Med Chem 92: 839-65 (2015) Article DOI: 10.1016/j.ejmech.2015.01.051 BindingDB Entry DOI: 10.7270/Q2NZ899Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase (Homo sapiens (Human)) | BDBM50121025 ((E)-1-[2,4-Dihydroxy-5-(3-methyl-but-2-enyl)-pheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Faculty of Medicine, University of Ni?, Bulevar Dr Zorana?in?i?a 81, 18000 Ni?, Serbia. Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase (unknown origin) using xanthine as substrate measured at every 1 mins up to 5 mins | Eur J Med Chem 135: 491-516 (2017) Article DOI: 10.1016/j.ejmech.2017.04.031 BindingDB Entry DOI: 10.7270/Q2VD71XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Middle East respiratory syndrome-related coronavir...) | BDBM50121025 ((E)-1-[2,4-Dihydroxy-5-(3-methyl-but-2-enyl)-pheny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn | Assay Description This is a review article. | Med Res Rev (2020) Article DOI: 10.1002/med.21724 BindingDB Entry DOI: 10.7270/Q2JS9ST6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL62 (Saccharomyces cerevisiae) | BDBM50121025 ((E)-1-[2,4-Dihydroxy-5-(3-methyl-but-2-enyl)-pheny...) | Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 5.30E+9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of baker's yeast alpha-glucosidase using p-nitrophenyl-alpha-D-glucopyranoside as substrate by spectrophotometry | Citation and Details Article DOI: 10.1007/s00044-011-9938-0 BindingDB Entry DOI: 10.7270/Q29W0JBG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine phosphatase 1B (Homo sapiens (Human)) | BDBM50121025 ((E)-1-[2,4-Dihydroxy-5-(3-methyl-but-2-enyl)-pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description The inhibitory activity of the compound tested against human Protein-tyrosine phosphatase 1B enzyme | Bioorg Med Chem Lett 12: 3387-90 (2002) BindingDB Entry DOI: 10.7270/Q2HH6JF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain-like protease (PLpro) (Human SARS coronavirus (SARS-CoV)) | BDBM50121025 ((E)-1-[2,4-Dihydroxy-5-(3-methyl-but-2-enyl)-pheny...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn | Assay Description This is a review article. | Med Res Rev (2020) Article DOI: 10.1002/med.21724 BindingDB Entry DOI: 10.7270/Q2JS9ST6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||