

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50104891 7-Hydroxy-4-phenyl-chromen-2-one::7-hydroxy-4-phenyl-2H-chromen-2-one::CHEMBL325841::Coumarin derivative, 3c::cid_5357479
SMILES: Oc1ccc2c(cc(=O)oc2c1)-c1ccccc1
InChI Key: InChIKey=IVJMJRRORVMRJJ-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrases; II & IX (Homo sapiens (Human)) | BDBM50104891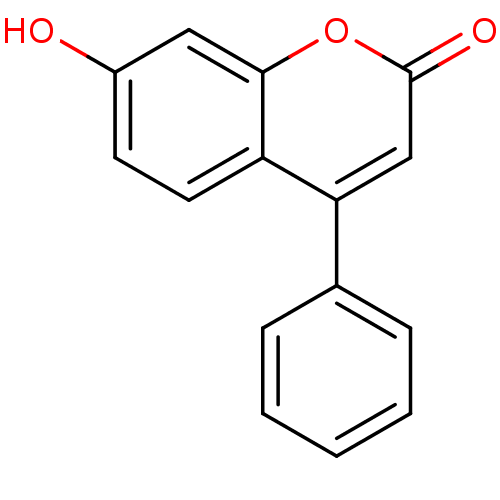 (7-Hydroxy-4-phenyl-chromen-2-one | 7-hydroxy-4-phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 9 incubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | Eur J Med Chem 143: 276-282 (2018) Article DOI: 10.1016/j.ejmech.2017.11.061 BindingDB Entry DOI: 10.7270/Q2DZ0BVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Homo sapiens (Human)) | BDBM50104891 (7-Hydroxy-4-phenyl-chromen-2-one | 7-hydroxy-4-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 12 incubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | Eur J Med Chem 143: 276-282 (2018) Article DOI: 10.1016/j.ejmech.2017.11.061 BindingDB Entry DOI: 10.7270/Q2DZ0BVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Homo sapiens (Human)) | BDBM50104891 (7-Hydroxy-4-phenyl-chromen-2-one | 7-hydroxy-4-phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 1 incubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | Eur J Med Chem 143: 276-282 (2018) Article DOI: 10.1016/j.ejmech.2017.11.061 BindingDB Entry DOI: 10.7270/Q2DZ0BVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50104891 (7-Hydroxy-4-phenyl-chromen-2-one | 7-hydroxy-4-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 incubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | Eur J Med Chem 143: 276-282 (2018) Article DOI: 10.1016/j.ejmech.2017.11.061 BindingDB Entry DOI: 10.7270/Q2DZ0BVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldose reductase (AR) (Homo sapiens (Human)) | BDBM50104891 (7-Hydroxy-4-phenyl-chromen-2-one | 7-hydroxy-4-phe...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition human recombinant aldose reductase 1 by spectrophotometric analysis | Bioorg Med Chem Lett 20: 5630-3 (2010) Article DOI: 10.1016/j.bmcl.2010.08.038 BindingDB Entry DOI: 10.7270/Q28052TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sorbitol dehydrogenase (Homo sapiens (Human)) | BDBM50104891 (7-Hydroxy-4-phenyl-chromen-2-one | 7-hydroxy-4-phe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition sorbitol dehydrogenase by spectrophotometric analysis | Bioorg Med Chem Lett 20: 5630-3 (2010) Article DOI: 10.1016/j.bmcl.2010.08.038 BindingDB Entry DOI: 10.7270/Q28052TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Butyrylcholinesterase (BuChE) (Equus caballus (Horse)) | BDBM50104891 (7-Hydroxy-4-phenyl-chromen-2-one | 7-hydroxy-4-phe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to ... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50104891 (7-Hydroxy-4-phenyl-chromen-2-one | 7-hydroxy-4-phe...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.58E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to 180... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Danio rerio) | BDBM50104891 (7-Hydroxy-4-phenyl-chromen-2-one | 7-hydroxy-4-phe...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.03E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nis Curated by ChEMBL | Assay Description Inhibition of zebrafish embryo tyrosinase assessed as melanin production after 48 hrs | Bioorg Med Chem 25: 6286-6296 (2017) Article DOI: 10.1016/j.bmc.2017.09.021 BindingDB Entry DOI: 10.7270/Q28G8P7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Danio rerio) | BDBM50104891 (7-Hydroxy-4-phenyl-chromen-2-one | 7-hydroxy-4-phe...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nis Curated by ChEMBL | Assay Description Inhibition of zebrafish embryo tyrosinase assessed as residual activity after 48 hrs | Bioorg Med Chem 25: 6286-6296 (2017) Article DOI: 10.1016/j.bmc.2017.09.021 BindingDB Entry DOI: 10.7270/Q28G8P7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 3 (Homo sapiens (Human)) | BDBM50104891 (7-Hydroxy-4-phenyl-chromen-2-one | 7-hydroxy-4-phe...) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human 17beta-HSD3 expressed in HeLa cells | Bioorg Med Chem Lett 20: 272-5 (2010) Article DOI: 10.1016/j.bmcl.2009.10.111 BindingDB Entry DOI: 10.7270/Q2SF2W8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Homo sapiens (Human)) | BDBM50104891 (7-Hydroxy-4-phenyl-chromen-2-one | 7-hydroxy-4-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of hamster liver aldehyde dehydrogenase ALDH-2 | J Med Chem 44: 3320-8 (2001) BindingDB Entry DOI: 10.7270/Q2CC0ZZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-structural protein 5B (NS5B) (Hepatitis C virus genotype 1b (isolate BK) (HCV)) | BDBM50104891 (7-Hydroxy-4-phenyl-chromen-2-one | 7-hydroxy-4-phe...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.16E+4 | n/a | n/a | n/a | n/a | 7.0 | 30 |
UMDNJ-New Jersey Medical School | Assay Description Inhibition assay using HCV NS5B. | Chem Biol Drug Des 81: 607-14 (2013) Article DOI: 10.1111/cbdd.12105 BindingDB Entry DOI: 10.7270/Q2R78CT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hsf1 protein (Mus musculus) | BDBM50104891 (7-Hydroxy-4-phenyl-chromen-2-one | 7-hydroxy-4-phe...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | n/a | 1.17E+5 | n/a | n/a | n/a | n/a |
Broad Institute Curated by PubChem BioAssay | Assay Description Keywords: Heat Shock Factor-1 (HSF-1), Stress Response, MG132, NIH3T3, Luminescence Assay Overview: Modified NIH3T3, transformed to express firefly... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2MW2FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50104891 (7-Hydroxy-4-phenyl-chromen-2-one | 7-hydroxy-4-phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | n/a | 2.46E+4 | n/a | n/a | n/a | n/a |
Broad Institute Curated by PubChem BioAssay | Assay Description Keywords: GSK3beta, dose response, kinase, inhibition, HTS Assay Overview: The glycogen synthase kinase-3 beta (GSK-3b) is a known master regulator f... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2TX3CTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||