

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50323214 6-[4-(5,7-Dihydroxy-4-oxo-4H-chromen-2-yl)-phenoxy]-5,7-dihydroxy-2-(4-hydroxy-phenyl)-chromen-4-one::CHEMBL291426::HINOKIFLAVONE
SMILES: Oc1ccc(cc1)-c1cc(=O)c2c(O)c(Oc3ccc(cc3)-c3cc(O)c4c(cc(O)cc4=O)o3)c(O)cc2o1
InChI Key: InChIKey=DWXKOQIOXVVGJJ-UHFFFAOYSA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50323214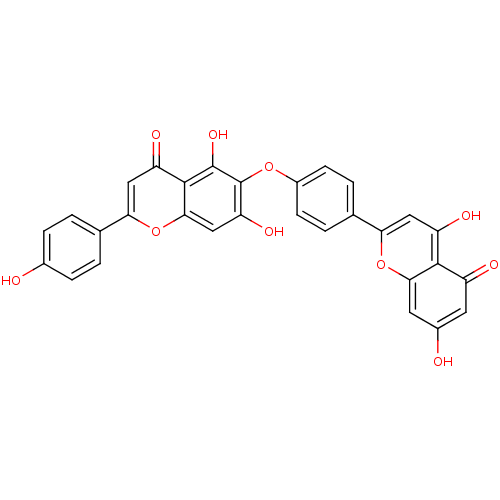 (6-[4-(5,7-Dihydroxy-4-oxo-4H-chromen-2-yl)-phenoxy...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shujitsu University Curated by ChEMBL | Assay Description Inhibition of BACE1 after 60 mins by FRET assay | Bioorg Med Chem Lett 20: 4558-60 (2010) Article DOI: 10.1016/j.bmcl.2010.06.021 BindingDB Entry DOI: 10.7270/Q2GT5P46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50323214 (6-[4-(5,7-Dihydroxy-4-oxo-4H-chromen-2-yl)-phenoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human Cathepsin K | Bioorg Med Chem Lett 16: 6178-80 (2006) Article DOI: 10.1016/j.bmcl.2006.09.042 BindingDB Entry DOI: 10.7270/Q2C25069 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50323214 (6-[4-(5,7-Dihydroxy-4-oxo-4H-chromen-2-yl)-phenoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human amyloid beta (1 to 40) assessed as reduction in aggregation measured after 24 hrs by ThT fluorescence assay | Bioorg Med Chem Lett 29: 1994-1997 (2019) Article DOI: 10.1016/j.bmcl.2019.05.020 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50323214 (6-[4-(5,7-Dihydroxy-4-oxo-4H-chromen-2-yl)-phenoxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Buenos Aires Curated by ChEMBL | Assay Description Inhibition of Influenza A PR/8/34 H1N1 virus neuraminidase activity by MUN-ANA substrate based fluorimetric assay | Eur J Med Chem 45: 1724-30 (2010) Article DOI: 10.1016/j.ejmech.2010.01.005 BindingDB Entry DOI: 10.7270/Q27P926M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50323214 (6-[4-(5,7-Dihydroxy-4-oxo-4H-chromen-2-yl)-phenoxy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
MediChem Research, Inc. Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase p66/p51 expressed in Escherichia coli | J Nat Prod 60: 884-8 (1997) Article DOI: 10.1021/np9700275 BindingDB Entry DOI: 10.7270/Q2JD50MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50323214 (6-[4-(5,7-Dihydroxy-4-oxo-4H-chromen-2-yl)-phenoxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.18E+10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Buenos Aires Curated by ChEMBL | Assay Description Inhibition of Influenza A PR/8/34 H1N1 virus neuraminidase activity by MUN-ANA substrate based fluorimetric assay | Eur J Med Chem 45: 1724-30 (2010) Article DOI: 10.1016/j.ejmech.2010.01.005 BindingDB Entry DOI: 10.7270/Q27P926M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||