

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50208612 (-)-kurarinone::(2S)-2-(2,4-dihydroxyphenyl)-7-hydroxy-5-methoxy-8-(5-methyl-2-(prop-1-en-2-yl)hex-4-enyl)chroman-4-one::CHEMBL243796::kurarinone
SMILES: [#6]-[#8]-c1cc(-[#8])c(-[#6]-[#6](-[#6]\[#6]=[#6](\[#6])-[#6])-[#6](-[#6])=[#6])c2-[#8]-[#6@@H](-[#6]-[#6](=O)-c12)-c1ccc(-[#8])cc1-[#8]
InChI Key: InChIKey=LTTQKYMNTNISSZ-KESSSICBSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50208612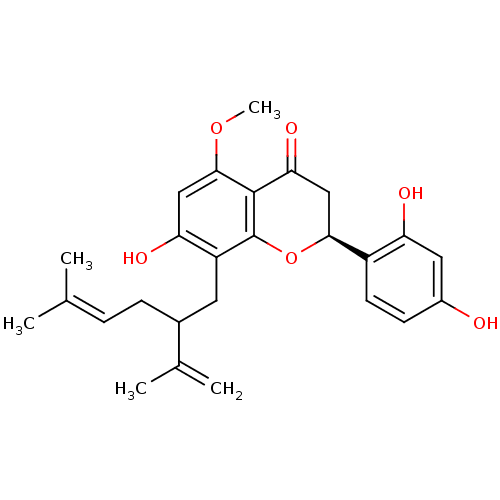 ((-)-kurarinone | (2S)-2-(2,4-dihydroxyphenyl)-7-hy...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human BACE1 | Bioorg Med Chem 16: 6669-74 (2008) Article DOI: 10.1016/j.bmc.2008.05.080 BindingDB Entry DOI: 10.7270/Q2HT2Q74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50208612 ((-)-kurarinone | (2S)-2-(2,4-dihydroxyphenyl)-7-hy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Suisan Kaisha, Ltd Curated by ChEMBL | Assay Description Inhibition of human SGLT2 expressed in COS1 cells assessed as [14C]methyl-alpha-D-glucopyranoside uptake | Bioorg Med Chem 15: 3445-9 (2007) Article DOI: 10.1016/j.bmc.2007.03.011 BindingDB Entry DOI: 10.7270/Q25X28N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (SGLT1) (Homo sapiens (Human)) | BDBM50208612 ((-)-kurarinone | (2S)-2-(2,4-dihydroxyphenyl)-7-hy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Suisan Kaisha, Ltd Curated by ChEMBL | Assay Description Inhibition of human SGLT1 expressed in COS1 cells assessed as [14C]methyl-alpha-D-glucopyranoside uptake | Bioorg Med Chem 15: 3445-9 (2007) Article DOI: 10.1016/j.bmc.2007.03.011 BindingDB Entry DOI: 10.7270/Q25X28N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacyl Glycerolacyltransferase 1 (DGAT-1) (Rattus norvegicus (rat)) | BDBM50208612 ((-)-kurarinone | (2S)-2-(2,4-dihydroxyphenyl)-7-hy...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dongguk University-Seoul Curated by ChEMBL | Assay Description Inhibition of DGAT in rat liver microsomes | Bioorg Med Chem Lett 22: 7456-60 (2012) Article DOI: 10.1016/j.bmcl.2012.10.046 BindingDB Entry DOI: 10.7270/Q2WH2R5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase-1 (COX-1) (Homo sapiens (Human)) | BDBM50208612 ((-)-kurarinone | (2S)-2-(2,4-dihydroxyphenyl)-7-hy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of COX1 | J Nat Prod 68: 985-91 (2005) Article DOI: 10.1021/np049655u BindingDB Entry DOI: 10.7270/Q27S7PN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50208612 ((-)-kurarinone | (2S)-2-(2,4-dihydroxyphenyl)-7-hy...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Estrogenic activity at human estrogen receptor expressing Saccharomyces cerevisiae carrying estrogen responsive sequence contatining plasmid assessed... | J Nat Prod 67: 1829-32 (2004) Article DOI: 10.1021/np040069a BindingDB Entry DOI: 10.7270/Q2SN09TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50208612 ((-)-kurarinone | (2S)-2-(2,4-dihydroxyphenyl)-7-hy...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.66E+3 | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Estrogenic activity at estrogen receptor in human Ishikawa Var-1 cells assessed as stimulation of alkaline phosphatase activity measured by metabolis... | J Nat Prod 67: 1829-32 (2004) Article DOI: 10.1021/np040069a BindingDB Entry DOI: 10.7270/Q2SN09TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50208612 ((-)-kurarinone | (2S)-2-(2,4-dihydroxyphenyl)-7-hy...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dongguk University-Seoul Curated by ChEMBL | Assay Description Inhibition of human macrophage ACAT expressed in human HepG2 cells using [14C]oleoyl-CoA as substrate incubated for 20 mins prior to substrate additi... | Eur J Med Chem 62: 515-25 (2013) Article DOI: 10.1016/j.ejmech.2013.01.020 BindingDB Entry DOI: 10.7270/Q29G5P5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||