 Found 8 hits for monomerid = 50240512
Found 8 hits for monomerid = 50240512 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Arachidonate 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50240512
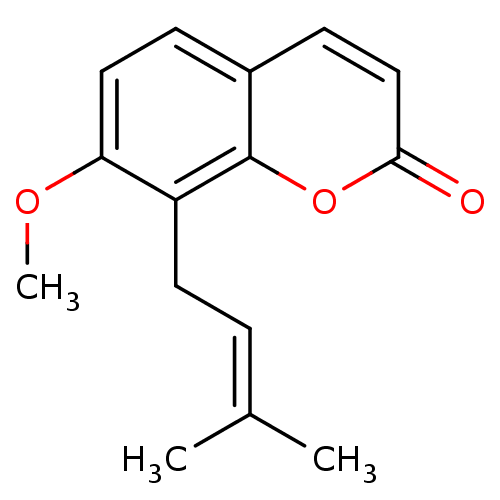
(7-Methoxy-8-(3-methyl-but-2-enyl)-chromen-2-one | ...)Show SMILES [#6]-[#8]-c1ccc2ccc(=O)oc2c1-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C15H16O3/c1-10(2)4-7-12-13(17-3)8-5-11-6-9-14(16)18-15(11)12/h4-6,8-9H,7H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich-Heine-Universität Düsseldorf
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase |
J Nat Prod 61: 347-50 (1998)
Article DOI: 10.1021/np970430b
BindingDB Entry DOI: 10.7270/Q2PN95D7 |
More data for this
Ligand-Target Pair | |
Cyclooxygenase-1 (COX-1)
(Homo sapiens (Human)) | BDBM50240512

(7-Methoxy-8-(3-methyl-but-2-enyl)-chromen-2-one | ...)Show SMILES [#6]-[#8]-c1ccc2ccc(=O)oc2c1-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C15H16O3/c1-10(2)4-7-12-13(17-3)8-5-11-6-9-14(16)18-15(11)12/h4-6,8-9H,7H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich-Heine-Universität Düsseldorf
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Nat Prod 61: 347-50 (1998)
Article DOI: 10.1021/np970430b
BindingDB Entry DOI: 10.7270/Q2PN95D7 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50240512

(7-Methoxy-8-(3-methyl-but-2-enyl)-chromen-2-one | ...)Show SMILES [#6]-[#8]-c1ccc2ccc(=O)oc2c1-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C15H16O3/c1-10(2)4-7-12-13(17-3)8-5-11-6-9-14(16)18-15(11)12/h4-6,8-9H,7H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 using Rh-EVNLDAEFK as substrate after 60 mins by fluorescence quenching assay |
Bioorg Med Chem 20: 784-8 (2012)
Article DOI: 10.1016/j.bmc.2011.12.002
BindingDB Entry DOI: 10.7270/Q2F47PKK |
More data for this
Ligand-Target Pair | |
GABA-A receptor; alpha-1/beta-2/gamma-2
(Homo sapiens (Human)) | BDBM50240512

(7-Methoxy-8-(3-methyl-but-2-enyl)-chromen-2-one | ...)Show SMILES [#6]-[#8]-c1ccc2ccc(=O)oc2c1-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C15H16O3/c1-10(2)4-7-12-13(17-3)8-5-11-6-9-14(16)18-15(11)12/h4-6,8-9H,7H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of human GABA-A alpha1beta2gamma2S receptor expressed in Xenopus laevis oocytes assessed as increase in GABA-induced c... |
Eur J Med Chem 171: 434-461 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.043 |
More data for this
Ligand-Target Pair | |
Glyceraldehyde-3-phosphate dehydrogenase liver
(Homo sapiens (Human)) | BDBM50240512

(7-Methoxy-8-(3-methyl-but-2-enyl)-chromen-2-one | ...)Show SMILES [#6]-[#8]-c1ccc2ccc(=O)oc2c1-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C15H16O3/c1-10(2)4-7-12-13(17-3)8-5-11-6-9-14(16)18-15(11)12/h4-6,8-9H,7H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.09E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de Minas Gerais
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glyceraldehyde-3-phosphate dehydrogenase was determined as log 1/IC50 |
Bioorg Med Chem Lett 14: 2199-204 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.025
BindingDB Entry DOI: 10.7270/Q20K2810 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase (cyclooxygenase)
(Ovis aries (Sheep)) | BDBM50240512

(7-Methoxy-8-(3-methyl-but-2-enyl)-chromen-2-one | ...)Show SMILES [#6]-[#8]-c1ccc2ccc(=O)oc2c1-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C15H16O3/c1-10(2)4-7-12-13(17-3)8-5-11-6-9-14(16)18-15(11)12/h4-6,8-9H,7H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using arachidonic acid as substrate pretreated for 3 mins followed by substrate addition measured immediately |
J Nat Prod 80: 2472-2477 (2017)
Article DOI: 10.1021/acs.jnatprod.7b00322
BindingDB Entry DOI: 10.7270/Q29Z97DR |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase/G/H synthase 2
(Homo sapiens (Human)) | BDBM50240512

(7-Methoxy-8-(3-methyl-but-2-enyl)-chromen-2-one | ...)Show SMILES [#6]-[#8]-c1ccc2ccc(=O)oc2c1-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C15H16O3/c1-10(2)4-7-12-13(17-3)8-5-11-6-9-14(16)18-15(11)12/h4-6,8-9H,7H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan State University
Curated by ChEMBL
| Assay Description
Antagonistic potency for NK-3 receptor was determined in vitro using isolated rat portal vein. |
J Nat Prod 80: 2472-2477 (2017)
Article DOI: 10.1021/acs.jnatprod.7b00322
BindingDB Entry DOI: 10.7270/Q29Z97DR |
More data for this
Ligand-Target Pair | |
Exportin-1
(Homo sapiens (Human)) | BDBM50240512

(7-Methoxy-8-(3-methyl-but-2-enyl)-chromen-2-one | ...)Show SMILES [#6]-[#8]-c1ccc2ccc(=O)oc2c1-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C15H16O3/c1-10(2)4-7-12-13(17-3)8-5-11-6-9-14(16)18-15(11)12/h4-6,8-9H,7H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Inhibition of CRM1-mediated hemagglutinin-tagged HIV1 Rev protein nuclear export in human HeLa cells assessed as nucleus localization after 12 hrs by... |
Bioorg Med Chem Lett 20: 3717-20 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.081
BindingDB Entry DOI: 10.7270/Q21G0MF1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data