

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM10774 (3R)-3-[methyl(prop-2-yn-1-yl)amino]-2,3-dihydro-1H-inden-5-yl N-ethyl-N-methylcarbamate::6-(N-methyl-N-ethyl-carbamoyloxy)-N-methyl-N-propargyl--1(R)-aminoindan::Aminoindan deriv. (R)19b::N-propargylaminoindan (R)19b::R-M6CPAI::rasagiline analog
SMILES: CCN(C)C(=O)Oc1ccc2CC[C@@H](N(C)CC#C)c2c1
InChI Key: InChIKey=UJHNPGCWWGEWLU-MRXNPFEDSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM10774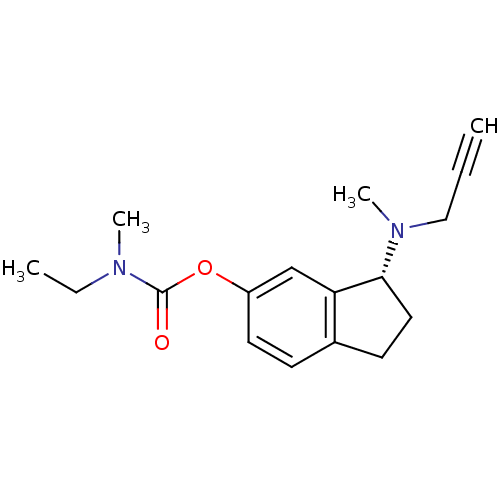 ((3R)-3-[methyl(prop-2-yn-1-yl)amino]-2,3-dihydro-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.37E+5 | -5.27 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Pavia | Assay Description MAO A and MAO B activities were determined spectrophotometrically at 316 nm and 250 nm using kynuramine and benzylamine as substrates, respectively. ... | J Med Chem 48: 8148-54 (2005) Article DOI: 10.1021/jm0506266 BindingDB Entry DOI: 10.7270/Q2T151WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase (flavin-containing) A (Homo sapiens (Human)) | BDBM10774 ((3R)-3-[methyl(prop-2-yn-1-yl)amino]-2,3-dihydro-1...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.95E+5 | -4.81 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Pavia | Assay Description MAO A and MAO B activities were determined spectrophotometrically at 316 nm and 250 nm using kynuramine and benzylamine as substrates, respectively. ... | J Med Chem 48: 8148-54 (2005) Article DOI: 10.1021/jm0506266 BindingDB Entry DOI: 10.7270/Q2T151WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoamine oxidase (Rattus norvegicus (rat)) | BDBM10774 ((3R)-3-[methyl(prop-2-yn-1-yl)amino]-2,3-dihydro-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description Inhibition of MAO activity was determined by a radiometric procedure from Tipton and Youdim. Homogenized rat brain was used as the source of enzymes.... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Butyrylcholinesterase (BChE) (Equus caballus (Horse)) | BDBM10774 ((3R)-3-[methyl(prop-2-yn-1-yl)amino]-2,3-dihydro-1...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoamine Oxidase Type B (MAO-B) (Rattus norvegicus (rat)) | BDBM10774 ((3R)-3-[methyl(prop-2-yn-1-yl)amino]-2,3-dihydro-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description Inhibition of MAO activity was determined by a radiometric procedure from Tipton and Youdim. Homogenized rat brain was used as the source of enzymes.... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10774 ((3R)-3-[methyl(prop-2-yn-1-yl)amino]-2,3-dihydro-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||