

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM12152 2-carboxymethylbenzenesulfonamide 5::CHEMBL148680::methyl 2-sulfamoylbenzoate
SMILES: COC(=O)c1ccccc1S(N)(=O)=O
InChI Key: InChIKey=VSOOBQALJVLTBH-UHFFFAOYSA-N
PDB links: 8 PDB IDs contain this monomer as substructures. 9 PDB IDs contain inhibitors having a similarity of 90% to this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM12152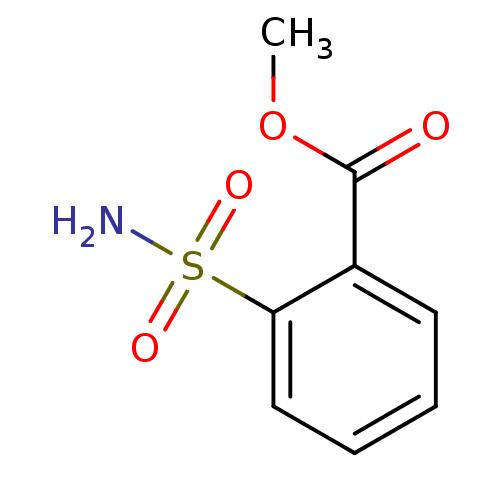 (2-carboxymethylbenzenesulfonamide 5 | CHEMBL148680...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 48: 2121-5 (2005) Article DOI: 10.1021/jm0494826 BindingDB Entry DOI: 10.7270/Q25X275C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM12152 (2-carboxymethylbenzenesulfonamide 5 | CHEMBL148680...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 48: 2121-5 (2005) Article DOI: 10.1021/jm0494826 BindingDB Entry DOI: 10.7270/Q25X275C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM12152 (2-carboxymethylbenzenesulfonamide 5 | CHEMBL148680...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 3.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 48: 2121-5 (2005) Article DOI: 10.1021/jm0494826 BindingDB Entry DOI: 10.7270/Q25X275C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic Anhydrase VA (Homo sapiens (Human)) | BDBM12152 (2-carboxymethylbenzenesulfonamide 5 | CHEMBL148680...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 5.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory concentration against human cystolic isozyme V of Carbonic anhydrase | Bioorg Med Chem Lett 14: 5703-7 (2004) Article DOI: 10.1016/j.bmcl.2004.07.085 BindingDB Entry DOI: 10.7270/Q23N22VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM12152 (2-carboxymethylbenzenesulfonamide 5 | CHEMBL148680...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 7.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory concentration against human cystolic isozyme II of Carbonic anhydrase | Bioorg Med Chem Lett 14: 5703-7 (2004) Article DOI: 10.1016/j.bmcl.2004.07.085 BindingDB Entry DOI: 10.7270/Q23N22VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||