

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM12941 4-({[4-(Aminosulfonyl)benzoyl]oxy}methyl-1-(beta-D-maltosyl)-1H-1,2,3-triazole::CHEMBL217288::Glycoconjugate Benzene Sulfonamide 3ff::{1-[(2R,3R,4R,5S,6R)-3,4-dihydroxy-6-(hydroxymethyl)-5-{[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}oxan-2-yl]-1H-1,2,3-triazol-4-yl}methyl 4-sulfamoylbenzoate
SMILES: NS(=O)(=O)c1ccc(cc1)C(=O)OCc1cn(nn1)[C@@H]1O[C@H](CO)[C@@H](O[C@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)[C@H](O)[C@H]1O
InChI Key: InChIKey=WOKLSLIENJMQHZ-XBTKAFEJSA-N
Data: 5 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM12941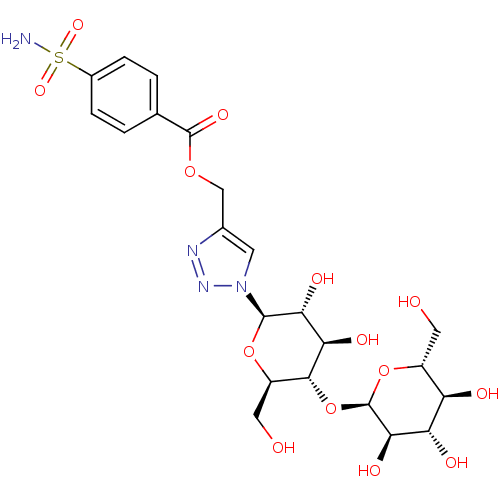 (4-({[4-(Aminosulfonyl)benzoyl]oxy}methyl-1-(beta-D...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA12 by CO2 hydration assay | Bioorg Med Chem Lett 17: 987-92 (2007) Article DOI: 10.1016/j.bmcl.2006.11.046 BindingDB Entry DOI: 10.7270/Q2JD4XMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM12941 (4-({[4-(Aminosulfonyl)benzoyl]oxy}methyl-1-(beta-D...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 49: 6539-48 (2006) Article DOI: 10.1021/jm060967z BindingDB Entry DOI: 10.7270/Q2X928HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic Anhydrase XIV (Homo sapiens (Human)) | BDBM12941 (4-({[4-(Aminosulfonyl)benzoyl]oxy}methyl-1-(beta-D...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 10.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA14 by CO2 hydration assay | Bioorg Med Chem Lett 17: 987-92 (2007) Article DOI: 10.1016/j.bmcl.2006.11.046 BindingDB Entry DOI: 10.7270/Q2JD4XMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM12941 (4-({[4-(Aminosulfonyl)benzoyl]oxy}methyl-1-(beta-D...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 49: 6539-48 (2006) Article DOI: 10.1021/jm060967z BindingDB Entry DOI: 10.7270/Q2X928HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM12941 (4-({[4-(Aminosulfonyl)benzoyl]oxy}methyl-1-(beta-D...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 49: 6539-48 (2006) Article DOI: 10.1021/jm060967z BindingDB Entry DOI: 10.7270/Q2X928HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||