

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM13089 CGS 27023A Analog 17::N-hydroxy-2-{[4-(3-methylbutoxy)benzene](2-methylpropyl)sulfonamido}acetamide
SMILES: CC(C)CCOc1ccc(cc1)S(=O)(=O)N(CC(C)C)CC(=O)NO
InChI Key: InChIKey=GATKQMPWFHNBFF-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13089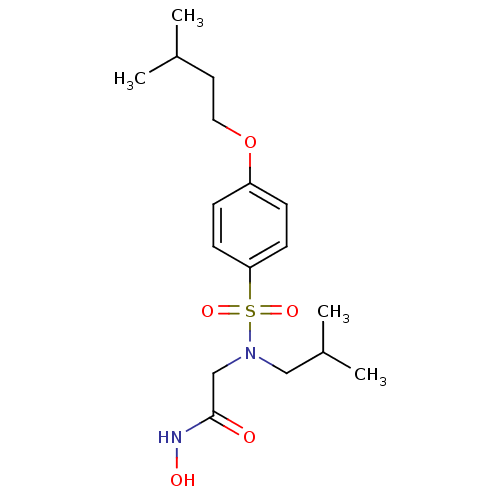 (CGS 27023A Analog 17 | N-hydroxy-2-{[4-(3-methylbu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pomona College Curated by ChEMBL | Assay Description Inhibition of human recombinant MMP3 | Bioorg Med Chem 15: 2223-68 (2007) Article DOI: 10.1016/j.bmc.2007.01.011 BindingDB Entry DOI: 10.7270/Q2571DBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13089 (CGS 27023A Analog 17 | N-hydroxy-2-{[4-(3-methylbu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 78 | -10.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||