

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM17298 4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclopropyl]-1H-1,3-benzodiazol-2-yl}methyl)amino]benzene-1-carboximidamide::BIBM1015
SMILES: Cn1c(CNc2ccc(cc2)C(N)=N)nc2cc(ccc12)C1(CC1)C(=O)N1CCCC1
InChI Key: InChIKey=HGNSKHRWYAJRBQ-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM17298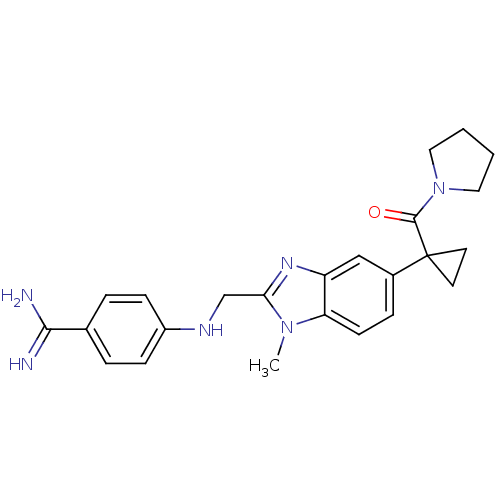 (4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | -11.1 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM17298 (4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | -10.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypsin (Bos taurus (bovine)) | BDBM17298 (4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 102 | -9.91 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM17298 (4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 6.50E+3 | -7.36 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM17298 (4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 8.20E+3 | -7.21 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM17298 (4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30E+4 | -6.93 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM17298 (4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | >3.00E+4 | >-6.41 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM17298 (4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | >4.00E+4 | >-6.24 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG | Assay Description For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... | Structure 9: 29-37 (2001) Article DOI: 10.1016/s0969-2126(00)00551-7 BindingDB Entry DOI: 10.7270/Q2028PTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17298 (4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D Curated by ChEMBL | Assay Description Inhibition of factor-10a (unknown origin) | J Med Chem 56: 9441-56 (2014) Article DOI: 10.1021/jm4005835 BindingDB Entry DOI: 10.7270/Q20K2B0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||