

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM181118 US9138393, Histidine::US9144538, Histidine
SMILES: N[C@@H](Cc1c[nH]cn1)C(O)=O
InChI Key:
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM181118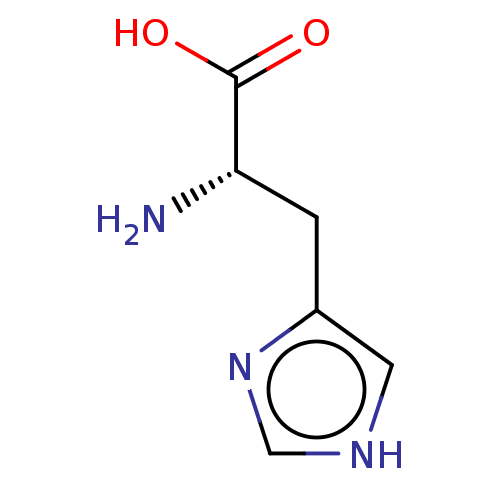 (US9138393, Histidine | US9144538, Histidine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Procter & Gamble Company US Patent | Assay Description A commercially available P450-GLO Assay kit (Promega Corporation, Madison Wis.) is used to screen various compounds for CYP3A4A inhibition activity. ... | US Patent US9138393 (2015) BindingDB Entry DOI: 10.7270/Q2GF0S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Large neutral amino acids transporter small subunit 1 (Homo sapiens (Human)) | BDBM181118 (US9138393, Histidine | US9144538, Histidine) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska at Kearney Curated by ChEMBL | Assay Description Cis-inhibition of human LAT1 expressed in TREx HEK293 cells assessed as inhibition of [3H]-gabapentin uptake preincubated for 3 mins at 37 degC follo... | Bioorg Med Chem Lett 29: 2254-2258 (2019) Article DOI: 10.1016/j.bmcl.2019.06.033 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Large neutral amino acids transporter small subunit 1 (Homo sapiens (Human)) | BDBM181118 (US9138393, Histidine | US9144538, Histidine) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Cis-inhibition of human LAT1 expressed in TREx HEK293 cells at 200 uM assessed as inhibition of [3H]-gabapentin uptake preincubated for 3 mins at 37 ... | J Med Chem 61: 7358-7373 (2018) Article DOI: 10.1021/acs.jmedchem.8b01007 BindingDB Entry DOI: 10.7270/Q2XK8J66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM181118 (US9138393, Histidine | US9144538, Histidine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Procter & Gamble Company US Patent | Assay Description Cytochrome P450 is a large and diverse group of enzymes that catalyze the oxidation of organic substances. Some members of the CYP family contribute ... | US Patent US9144538 (2015) BindingDB Entry DOI: 10.7270/Q22806DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||