

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM19492 (2R)-2-{[(1S)-1-(4-bromophenyl)-2,2,2-trifluoroethyl]amino}-N-(1-cyanocyclopropyl)-3-methanesulfonylpropanamide::CHEMBL400387::trifluoroethylamine inhibitor, 2
SMILES: CS(=O)(=O)C[C@H](N[C@@H](c1ccc(Br)cc1)C(F)(F)F)C(=O)NC1(CC1)C#N
InChI Key: InChIKey=QHDGGHMKRWZOAZ-STQMWFEESA-N
Data: 12 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin S (Homo sapiens (Human)) | BDBM19492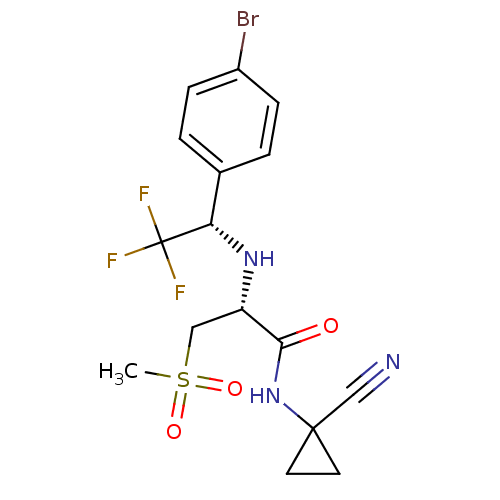 ((2R)-2-{[(1S)-1-(4-bromophenyl)-2,2,2-trifluoroeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 17: 4929-33 (2007) Article DOI: 10.1016/j.bmcl.2007.06.023 BindingDB Entry DOI: 10.7270/Q26W98C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM19492 ((2R)-2-{[(1S)-1-(4-bromophenyl)-2,2,2-trifluoroeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 282 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 17: 4929-33 (2007) Article DOI: 10.1016/j.bmcl.2007.06.023 BindingDB Entry DOI: 10.7270/Q26W98C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19492 ((2R)-2-{[(1S)-1-(4-bromophenyl)-2,2,2-trifluoroeth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 17: 4929-33 (2007) Article DOI: 10.1016/j.bmcl.2007.06.023 BindingDB Entry DOI: 10.7270/Q26W98C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19492 ((2R)-2-{[(1S)-1-(4-bromophenyl)-2,2,2-trifluoroeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 427 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 17: 4929-33 (2007) Article DOI: 10.1016/j.bmcl.2007.06.023 BindingDB Entry DOI: 10.7270/Q26W98C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Mus musculus) | BDBM19492 ((2R)-2-{[(1S)-1-(4-bromophenyl)-2,2,2-trifluoroeth...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of neutrophil elastase activation in beta-estradiol differentiated mouse EcoM-G cells after 24 hrs | J Biol Chem 282: 20836-46 (2007) Article DOI: 10.1074/jbc.M702615200 BindingDB Entry DOI: 10.7270/Q2125TJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin G (Homo sapiens (Human)) | BDBM19492 ((2R)-2-{[(1S)-1-(4-bromophenyl)-2,2,2-trifluoroeth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Blockade of cathepsin G processing in human U937 cells by densitometry | J Biol Chem 282: 20836-46 (2007) Article DOI: 10.1074/jbc.M702615200 BindingDB Entry DOI: 10.7270/Q2125TJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase I (Homo sapiens (Human)) | BDBM19492 ((2R)-2-{[(1S)-1-(4-bromophenyl)-2,2,2-trifluoroeth...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin C after 10 mins | J Biol Chem 282: 20836-46 (2007) Article DOI: 10.1074/jbc.M702615200 BindingDB Entry DOI: 10.7270/Q2125TJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM19492 ((2R)-2-{[(1S)-1-(4-bromophenyl)-2,2,2-trifluoroeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin B after 10 mins | J Biol Chem 282: 20836-46 (2007) Article DOI: 10.1074/jbc.M702615200 BindingDB Entry DOI: 10.7270/Q2125TJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19492 ((2R)-2-{[(1S)-1-(4-bromophenyl)-2,2,2-trifluoroeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin L after 10 mins | J Biol Chem 282: 20836-46 (2007) Article DOI: 10.1074/jbc.M702615200 BindingDB Entry DOI: 10.7270/Q2125TJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19492 ((2R)-2-{[(1S)-1-(4-bromophenyl)-2,2,2-trifluoroeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S after 10 mins | J Biol Chem 282: 20836-46 (2007) Article DOI: 10.1074/jbc.M702615200 BindingDB Entry DOI: 10.7270/Q2125TJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM19492 ((2R)-2-{[(1S)-1-(4-bromophenyl)-2,2,2-trifluoroeth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin H after 10 mins | J Biol Chem 282: 20836-46 (2007) Article DOI: 10.1074/jbc.M702615200 BindingDB Entry DOI: 10.7270/Q2125TJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin G (Mus musculus) | BDBM19492 ((2R)-2-{[(1S)-1-(4-bromophenyl)-2,2,2-trifluoroeth...) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of cathepsin G activation in beta-estradiol differentiated mouse EcoM-G cells after 24 hrs | J Biol Chem 282: 20836-46 (2007) Article DOI: 10.1074/jbc.M702615200 BindingDB Entry DOI: 10.7270/Q2125TJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||