

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM20182 (1S,2R,5S,10S,11S,14R,15R)-14-[(2R)-6-hydroxy-6-methylheptan-2-yl]-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-7-en-5-ol::25-Hydroxycholesterol (25OH)::25-hydroxycholesterol
SMILES: [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC=C4C[C@@]([H])(O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCCC(C)(C)O
InChI Key: InChIKey=INBGSXNNRGWLJU-ZHHJOTBYSA-N
PDB links: 9 PDB IDs match this monomer. 1 PDB ID contains this monomer as substructures. 1 PDB ID contains inhibitors having a similarity of 90% to this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20182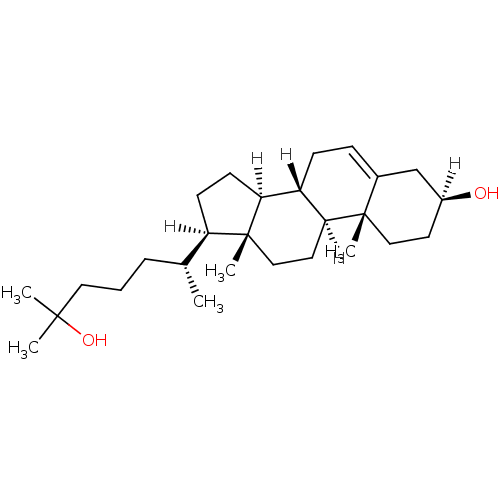 ((1S,2R,5S,10S,11S,14R,15R)-14-[(2R)-6-hydroxy-6-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.16E+3 | n/a | n/a | 7.5 | 22 |
Dartmouth College | Assay Description The LXR LiSA measures the ligand-dependent recruitment of a 25 amino acid fragment of the steroid receptor coactivator 1 (SRC1) to the ligand-binding... | J Med Chem 44: 886-97 (2001) Article DOI: 10.1021/jm0004749 BindingDB Entry DOI: 10.7270/Q2Z899P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| OSBP-related protein 4 (ORP4L) (Homo sapiens (Human)) | BDBM20182 ((1S,2R,5S,10S,11S,14R,15R)-14-[(2R)-6-hydroxy-6-me...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 17 | n/a | n/a | n/a | 7.4 | 20 |
Dalhousie University | Assay Description Recombinant OSBP, ORP4L, or ORP4S (8 pmol) was incubated in 75 μl of binding buffer (10 mM HEPES (pH 7.4), 150 mM KCl, 2% (w/v) polyvinyl alcohol)... | J Biol Chem 289: 15705-17 (2014) Article DOI: 10.1074/jbc.M114.571216 BindingDB Entry DOI: 10.7270/Q2N29VTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| OSBP-related protein 4 (ORP4S) (Homo sapiens (Human)) | BDBM20182 ((1S,2R,5S,10S,11S,14R,15R)-14-[(2R)-6-hydroxy-6-me...) | GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 23 | n/a | n/a | n/a | 7.4 | 20 |
Dalhousie University | Assay Description Recombinant OSBP, ORP4L, or ORP4S (8 pmol) was incubated in 75 μl of binding buffer (10 mM HEPES (pH 7.4), 150 mM KCl, 2% (w/v) polyvinyl alcohol)... | J Biol Chem 289: 15705-17 (2014) Article DOI: 10.1074/jbc.M114.571216 BindingDB Entry DOI: 10.7270/Q2N29VTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (RORC) (Homo sapiens (Human)) | BDBM20182 ((1S,2R,5S,10S,11S,14R,15R)-14-[(2R)-6-hydroxy-6-me...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]25-hydroxycholesterol from human RORc-LBD expressed in bacterial expression system after 3 hrs by scintillation counting analysis | Bioorg Med Chem Lett 24: 5769-76 (2014) Article DOI: 10.1016/j.bmcl.2014.10.037 BindingDB Entry DOI: 10.7270/Q2125V8F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Niemann-Pick C1-like protein 1 (Homo sapiens (Human)) | BDBM20182 ((1S,2R,5S,10S,11S,14R,15R)-14-[(2R)-6-hydroxy-6-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Binding affinity to FLAG/tGFP-tagged NPC1 I1061T mutant (unknown origin) expressed in HEK293 cells assessed as localization after 24 hrs by fluoresce... | Bioorg Med Chem Lett 24: 3480-5 (2014) Article DOI: 10.1016/j.bmcl.2014.05.064 BindingDB Entry DOI: 10.7270/Q2HX1F8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol regulatory element-binding protein 2 (Homo sapiens (Human)) | BDBM20182 ((1S,2R,5S,10S,11S,14R,15R)-14-[(2R)-6-hydroxy-6-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of SREBP2 activation expressed in CHO-K1 cells cotransfected with pSRE-Luc plasmid assessed as inhibition of luciferase expression after 2... | J Med Chem 54: 4923-7 (2011) Article DOI: 10.1021/jm200304y BindingDB Entry DOI: 10.7270/Q2NG4R0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HMG-CoA reductase (Homo sapiens (Human)) | BDBM20182 ((1S,2R,5S,10S,11S,14R,15R)-14-[(2R)-6-hydroxy-6-me...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 630 | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Induction of human HMGCR-dCat-ELuc degradation expressed in HEK293 cells assessed as reduction in luciferase activity after 4 hrs by luciferase repor... | Bioorg Med Chem 28: (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (RORC) (Homo sapiens (Human)) | BDBM20182 ((1S,2R,5S,10S,11S,14R,15R)-14-[(2R)-6-hydroxy-6-me...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description In vivo luteinising hormone inhibiting potency in rats is expressed as negative logarithm of the concentration (antagonist) | J Med Chem 61: 5794-5804 (2018) Article DOI: 10.1021/acs.jmedchem.7b01314 BindingDB Entry DOI: 10.7270/Q2M32Z85 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Alpha-crystallin B chain (Homo sapiens) | BDBM20182 ((1S,2R,5S,10S,11S,14R,15R)-14-[(2R)-6-hydroxy-6-me...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 320 | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of alpha-crystallin B R120G mutant (unknown origin)-induced intracellular protein aggregation expressed in human HeLa cells or HLE-B3 cell... | J Med Chem 61: 8693-8706 (2018) Article DOI: 10.1021/acs.jmedchem.8b00705 BindingDB Entry DOI: 10.7270/Q2JQ13NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (RORC) (Homo sapiens (Human)) | BDBM20182 ((1S,2R,5S,10S,11S,14R,15R)-14-[(2R)-6-hydroxy-6-me...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Activity at 6His-tagged human RORgamma LBD (262 to 507 residues) expressed in Escherichia coli BL21 (DE3) cells assessed as induction of biotinylated... | Bioorg Med Chem Lett 26: 4387-4393 (2016) Article DOI: 10.1016/j.bmcl.2016.08.012 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| NR1/NR2A (Homo sapiens (Human)) | BDBM20182 ((1S,2R,5S,10S,11S,14R,15R)-14-[(2R)-6-hydroxy-6-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
SAGE Therapeutics Curated by ChEMBL | Assay Description Positive allosteric modulation of recombinant human GluN1/GluN2A receptor stably expressed in HEK293 cells assessed as increase in glycine/L-glutamat... | J Med Chem 62: 7526-7542 (2019) Article DOI: 10.1021/acs.jmedchem.9b00591 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GluN1/GluN2B NMDA receptor (Homo sapiens (Human)) | BDBM20182 ((1S,2R,5S,10S,11S,14R,15R)-14-[(2R)-6-hydroxy-6-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
SAGE Therapeutics Curated by ChEMBL | Assay Description Positive allosteric modulation of recombinant human GluN1/GluN2B receptor stably expressed in HEK293 cells assessed as increase in glycine/L-glutamat... | J Med Chem 62: 7526-7542 (2019) Article DOI: 10.1021/acs.jmedchem.9b00591 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ITGAV/ITGB3 (Homo sapiens (Human)) | BDBM20182 ((1S,2R,5S,10S,11S,14R,15R)-14-[(2R)-6-hydroxy-6-me...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a |
Pliant Therapeutics Curated by ChEMBL | Assay Description Binding affinity to human integrin alphavbeta3 by surface plasmon resonance method | J Med Chem 63: 5675-5696 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA) (Homo sapiens (Human)) | BDBM20182 ((1S,2R,5S,10S,11S,14R,15R)-14-[(2R)-6-hydroxy-6-me...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Tested for its inhibitory activity against HMG-CoA reductase transcription in HepG2 cells | J Med Chem 37: 2343-51 (1994) BindingDB Entry DOI: 10.7270/Q2JH3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||