

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM23989 1,2,3,4-tetrahydronaphthalen-2-one::CHEMBL191664::dihydro-naphthalenone, 4e::dihydronaphthalenone
SMILES: O=C1CCc2ccccc2C1
InChI Key: InChIKey=KCKZIWSINLBROE-UHFFFAOYSA-N
Data: 8 IC50
PDB links: 6 PDB IDs contain this monomer as substructures. 6 PDB IDs contain inhibitors having a similarity of 90% to this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytosol aminopeptidase (Bos taurus (bovine)) | BDBM23989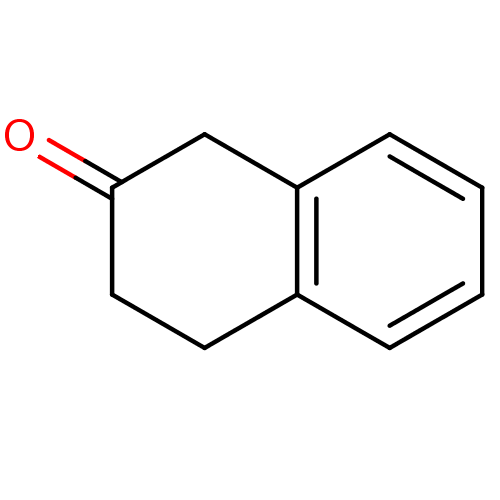 (1,2,3,4-tetrahydronaphthalen-2-one | CHEMBL191664 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | 8.0 | 30 |
ENSCMu | Assay Description Spectrophotometric assays were performed by monitoring hydrolysis of chromogenic substrate. The release of para-nitroaniline at 405 nm was measured t... | Bioorg Med Chem 14: 7241-57 (2006) Article DOI: 10.1016/j.bmc.2006.06.050 BindingDB Entry DOI: 10.7270/Q2N29V73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bacterial leucyl aminopeptidase (Vibrio proteolyticus) | BDBM23989 (1,2,3,4-tetrahydronaphthalen-2-one | CHEMBL191664 ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description Spectrophotometric assays were performed by monitoring hydrolysis of chromogenic substrate. The release of para-nitroaniline at 405 nm was measured t... | Bioorg Med Chem 14: 7241-57 (2006) Article DOI: 10.1016/j.bmc.2006.06.050 BindingDB Entry DOI: 10.7270/Q2N29V73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM23989 (1,2,3,4-tetrahydronaphthalen-2-one | CHEMBL191664 ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description Spectrophotometric assays were performed by monitoring hydrolysis of chromogenic substrate. The release of para-nitroaniline at 405 nm was measured t... | Bioorg Med Chem 14: 7241-57 (2006) Article DOI: 10.1016/j.bmc.2006.06.050 BindingDB Entry DOI: 10.7270/Q2N29V73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A5 (Mus musculus) | BDBM23989 (1,2,3,4-tetrahydronaphthalen-2-one | CHEMBL191664 ...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 9.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University Curated by ChEMBL | Assay Description Inhibition of mouse CYP2A5 | Eur J Med Chem 44: 1941-51 (2009) Article DOI: 10.1016/j.ejmech.2008.11.010 BindingDB Entry DOI: 10.7270/Q2PZ5B1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM23989 (1,2,3,4-tetrahydronaphthalen-2-one | CHEMBL191664 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kuopio Curated by ChEMBL | Assay Description Inhibitory concentration against human cytochrome P450 2A6 | J Med Chem 48: 440-9 (2005) Article DOI: 10.1021/jm049536b BindingDB Entry DOI: 10.7270/Q20C4WJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM23989 (1,2,3,4-tetrahydronaphthalen-2-one | CHEMBL191664 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University Curated by ChEMBL | Assay Description Inhibition of human CYP2A6 | Eur J Med Chem 44: 1941-51 (2009) Article DOI: 10.1016/j.ejmech.2008.11.010 BindingDB Entry DOI: 10.7270/Q2PZ5B1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A5 (Mus musculus) | BDBM23989 (1,2,3,4-tetrahydronaphthalen-2-one | CHEMBL191664 ...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kuopio Curated by ChEMBL | Assay Description Inhibitory concentration against mouse cytochrome P450 2A5 | J Med Chem 48: 440-9 (2005) Article DOI: 10.1021/jm049536b BindingDB Entry DOI: 10.7270/Q20C4WJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A4 hydrolase (Homo sapiens (Human)) | BDBM23989 (1,2,3,4-tetrahydronaphthalen-2-one | CHEMBL191664 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description Spectrophotometric assays were performed by monitoring hydrolysis of chromogenic substrate. The release of para-nitroaniline at 405 nm was measured t... | Bioorg Med Chem 14: 7241-57 (2006) Article DOI: 10.1016/j.bmc.2006.06.050 BindingDB Entry DOI: 10.7270/Q2N29V73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||