

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM247368 US9447092, Comparator 2, Example 57
SMILES: Cc1nn(C)c(C)c1-c1nc2c(N3CCN(Cc4cccnc4)CC3)c(Br)cnc2[nH]1
InChI Key: InChIKey=BMSHQZWQPJMOJN-UHFFFAOYSA-N
Data: 8 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aurora kinase A (Homo sapiens (Human)) | BDBM247368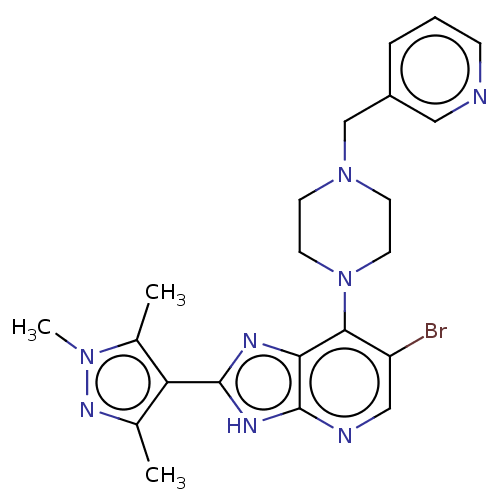 (US9447092, Comparator 2, Example 57) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Myc-tagged Aurora A was transfected in Hela cells using Lipofectamine LTX in 24 well plates, and 24 hours after transfection, cells were treated with... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM247368 (US9447092, Comparator 2, Example 57) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description All hERG percentage inhibitions at 10 uM compound concentration were determined by Millipore in a high-throughput cell-based electrophysiology assay ... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A (Homo sapiens (Human)) | BDBM247368 (US9447092, Comparator 2, Example 57) | PDB MMDB Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM247368 (US9447092, Comparator 2, Example 57) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM247368 (US9447092, Comparator 2, Example 57) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM247368 (US9447092, Comparator 2, Example 57) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM247368 (US9447092, Comparator 2, Example 57) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM247368 (US9447092, Comparator 2, Example 57) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||