

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM26132 (4-phenylphenyl)boranediol::Phenylboronic Acid, 11::Phenylboronic acid, 23
SMILES: OB(O)c1ccc(cc1)-c1ccccc1
InChI Key: InChIKey=XPEIJWZLPWNNOK-UHFFFAOYSA-N
PDB links: 1 PDB ID contains this monomer as substructures. 1 PDB ID contains inhibitors having a similarity of 90% to this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM26132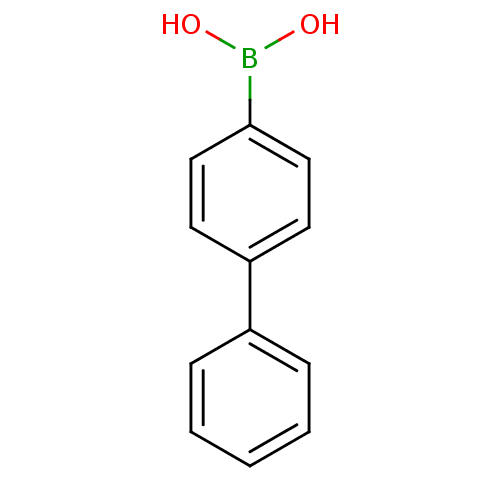 ((4-phenylphenyl)boranediol | Phenylboronic Acid, 1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human recombinant cytosolic carbonic anhydrase 1 preincubated for 15 mins by CO2 hydration stopped-flow assay | Bioorg Med Chem Lett 19: 2642-5 (2009) Article DOI: 10.1016/j.bmcl.2009.03.147 BindingDB Entry DOI: 10.7270/Q27D2V4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM26132 ((4-phenylphenyl)boranediol | Phenylboronic Acid, 1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human recombinant cytosolic carbonic anhydrase 2 preincubated for 15 mins by CO2 hydration stopped-flow assay | Bioorg Med Chem Lett 19: 2642-5 (2009) Article DOI: 10.1016/j.bmcl.2009.03.147 BindingDB Entry DOI: 10.7270/Q27D2V4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| beta-Carbonic Anhydrase (Candida albicans (Yeast)) | BDBM26132 ((4-phenylphenyl)boranediol | Phenylboronic Acid, 1...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of Candida albicans recombinant Carbonic anhydrase preincubated for 15 mins by CO2 hydration stopped-flow assay | Bioorg Med Chem Lett 19: 2642-5 (2009) Article DOI: 10.1016/j.bmcl.2009.03.147 BindingDB Entry DOI: 10.7270/Q27D2V4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-protein thioesterase 2 (Homo sapiens (Human)) | BDBM26132 ((4-phenylphenyl)boranediol | Phenylboronic Acid, 1...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Dortmund | Assay Description The enzyme activities were determined by measuring the release of fluorescent 6,8-difluoro-4-methylumbelliferone (DiFMU) by the APT hydrolysis of DiF... | Chembiochem 14: 115-22 (2013) Article DOI: 10.1002/cbic.201200571 BindingDB Entry DOI: 10.7270/Q2NS0SG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM26132 ((4-phenylphenyl)boranediol | Phenylboronic Acid, 1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Antagonist activity at recombinant human TRPV1 channel expressed in HEK293 cells assessed as inhibition of capsiacin-induced Ca2+ flux preincubated f... | Bioorg Med Chem Lett 26: 1401-5 (2016) BindingDB Entry DOI: 10.7270/Q24F1SM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-protein thioesterase 1 (Homo sapiens (Human)) | BDBM26132 ((4-phenylphenyl)boranediol | Phenylboronic Acid, 1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Dortmund | Assay Description The enzyme activities were determined by measuring the release of fluorescent 6,8-difluoro-4-methylumbelliferone (DiFMU) by the APT hydrolysis of DiF... | Chembiochem 14: 115-22 (2013) Article DOI: 10.1002/cbic.201200571 BindingDB Entry DOI: 10.7270/Q2NS0SG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM26132 ((4-phenylphenyl)boranediol | Phenylboronic Acid, 1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description The endpoint enzymatic assay was developed to quantify human recombinant MGL activity with 2-AG. The formation of arachidonic acid and depletion of ... | J Med Chem 51: 7057-60 (2008) Article DOI: 10.1021/jm801051t BindingDB Entry DOI: 10.7270/Q25D8Q58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (aa 30-579) (Rattus norvegicus (rat)) | BDBM26132 ((4-phenylphenyl)boranediol | Phenylboronic Acid, 1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | 7.4 | 37 |
University of Oxford | Assay Description [3H]Ethanolamine produced from [3H]AEA hydrolysis was used to calculate FAAH activity and was measured by scintillation counting of the aqueous phase... | J Med Chem 51: 7057-60 (2008) Article DOI: 10.1021/jm801051t BindingDB Entry DOI: 10.7270/Q25D8Q58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (aa 30-579) (Rattus norvegicus (rat)) | BDBM26132 ((4-phenylphenyl)boranediol | Phenylboronic Acid, 1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of rat brain FAAH assessed as hydrolysis of [14C]AEA to [14C]Ethanolamine incubated for 30 mins by scintillation counting method | Bioorg Med Chem Lett 26: 1401-5 (2016) BindingDB Entry DOI: 10.7270/Q24F1SM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||