

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM305765 2-chloro-N-(1-(5-(3- cyano-6- hydroxypyrazolo [1,5-a]pyridin- 4-yl)pyrazin-2-yl)-4- methylpiperidin-4- yl)-6- methylbenzamide::US10144734, Example 783::US10172845, Example 783::US10441581, Example 783::US10881652, Example 783
SMILES: Cc1cccc(Cl)c1C(=O)NC1(C)CCN(CC1)c1cnc(cn1)-c1cc(O)cn2ncc(C#N)c12
InChI Key: InChIKey=DIEDSUVCMVZYPX-UHFFFAOYSA-N
Data: 12 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM305765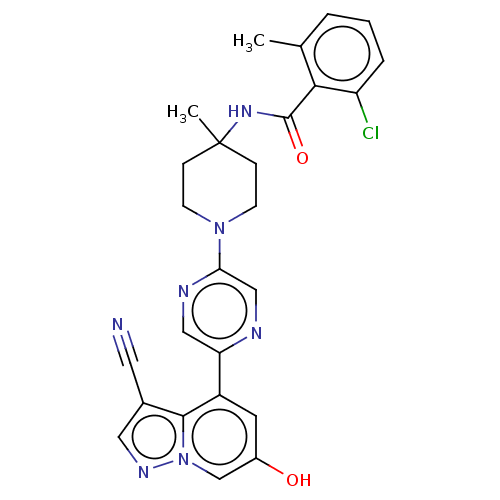 (2-chloro-N-(1-(5-(3- cyano-6- hydroxypyrazolo [1,5...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc. US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF KinEASE-TK assay techn... | US Patent US10144734 (2018) BindingDB Entry DOI: 10.7270/Q2251M8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RET kinase mutant (V804M) (Homo sapiens (Human)) | BDBM305765 (2-chloro-N-(1-(5-(3- cyano-6- hydroxypyrazolo [1,5...) | PDB GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc. US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF KinEASE-TK assay techn... | US Patent US10144734 (2018) BindingDB Entry DOI: 10.7270/Q2251M8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RET kinase mutant (G810R) (Homo sapiens (Human)) | BDBM305765 (2-chloro-N-(1-(5-(3- cyano-6- hydroxypyrazolo [1,5...) | PDB GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc. US Patent | Assay Description The potency of a compound inhibiting G81 OR mutant RET kinase was determined using CisBio's HTRF Kinease-TK assay technology. The assays containe... | US Patent US10144734 (2018) BindingDB Entry DOI: 10.7270/Q2251M8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RET Kinase (aa 658-1114) (Homo sapiens (Human)) | BDBM305765 (2-chloro-N-(1-(5-(3- cyano-6- hydroxypyrazolo [1,5...) | PDB GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc. US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF® KinEASE-TK assay tech... | US Patent US10172845 (2019) BindingDB Entry DOI: 10.7270/Q2M90BR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RET Kinase (V804M) (aa658-end) (Homo sapiens (Human)) | BDBM305765 (2-chloro-N-(1-(5-(3- cyano-6- hydroxypyrazolo [1,5...) | PDB GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc. US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF® KinEASE-TK assay tech... | US Patent US10172845 (2019) BindingDB Entry DOI: 10.7270/Q2M90BR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RET kinase mutant (G810R) (Homo sapiens (Human)) | BDBM305765 (2-chloro-N-(1-(5-(3- cyano-6- hydroxypyrazolo [1,5...) | PDB GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Array Biopharma Inc. US Patent | Assay Description The potency of a compound inhibiting G81 OR mutant RET kinase was determined using CisBio's HTRF Kinease-TK assay technology. The assays containe... | US Patent US10881652 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RET Kinase (V804M) (aa658-end) (Homo sapiens (Human)) | BDBM305765 (2-chloro-N-(1-(5-(3- cyano-6- hydroxypyrazolo [1,5...) | PDB GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Array Biopharma Inc. US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF KinEASE-TK assay techn... | US Patent US10441581 (2019) BindingDB Entry DOI: 10.7270/Q2DZ0BP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RET kinase mutant (G810R) (Homo sapiens (Human)) | BDBM305765 (2-chloro-N-(1-(5-(3- cyano-6- hydroxypyrazolo [1,5...) | PDB GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Array Biopharma Inc. US Patent | Assay Description The potency of a compound inhibiting G810R mutant RET kinase was determined using CisBio's HTRF Kinease-TK assay technology. The assays contained... | US Patent US10441581 (2019) BindingDB Entry DOI: 10.7270/Q2DZ0BP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RET kinase mutant (V804M) (Homo sapiens (Human)) | BDBM305765 (2-chloro-N-(1-(5-(3- cyano-6- hydroxypyrazolo [1,5...) | PDB GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Array Biopharma Inc. US Patent | Assay Description The potency of a compound inhibiting G810R mutant RET kinase was determined using CisBio's HTRF Kinease-TK assay technology. The assays contained... | US Patent US10441581 (2019) BindingDB Entry DOI: 10.7270/Q2DZ0BP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM305765 (2-chloro-N-(1-(5-(3- cyano-6- hydroxypyrazolo [1,5...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Array Biopharma Inc. US Patent | Assay Description Wildtype and V804M mutant: Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's ... | US Patent US10881652 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RET kinase mutant (V804M) (Homo sapiens (Human)) | BDBM305765 (2-chloro-N-(1-(5-(3- cyano-6- hydroxypyrazolo [1,5...) | PDB GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Array Biopharma Inc. US Patent | Assay Description Wildtype and V804M mutant: Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's ... | US Patent US10881652 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RET kinase mutant (G810R) (Homo sapiens (Human)) | BDBM305765 (2-chloro-N-(1-(5-(3- cyano-6- hydroxypyrazolo [1,5...) | PDB GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc. US Patent | Assay Description The potency of a compound inhibiting G810R mutant RET kinase was determined using CisBio's HTRF Kinease-TK assay technology. The assays contained... | US Patent US10172845 (2019) BindingDB Entry DOI: 10.7270/Q2M90BR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||