

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: COc1ccc(CC2CCN(CC2)C(=O)c2ccc(nc2)C(=O)NC2CCN(Cc3ccc(cc3)C#N)CC2)cc1
InChI Key: InChIKey=CXYVYGXMFQSTKH-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5'-AMP-activated protein kinase catalytic subunit alpha-2 (Homo sapiens (Human)) | BDBM327994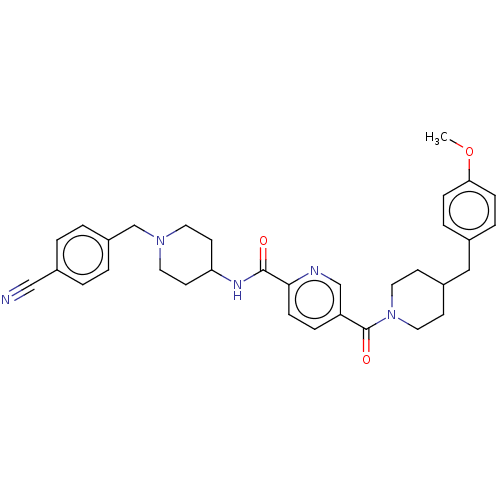 (N-(1-(4- cyanobenzyl)piperidin-4- yl)-5-(4-(4- met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <500 | n/a | n/a | n/a | n/a |
Rigel Pharmaceuticals, Inc. US Patent | Assay Description Compounds were assayed for their ability to activate AMPK using an enzyme-linked immunosorbent assay. Reagents and procedures for measuring AMPK acti... | US Patent US9663496 (2017) BindingDB Entry DOI: 10.7270/Q26975P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM327994 (N-(1-(4- cyanobenzyl)piperidin-4- yl)-5-(4-(4- met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116951 BindingDB Entry DOI: 10.7270/Q2HX1HM7 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM327994 (N-(1-(4- cyanobenzyl)piperidin-4- yl)-5-(4-(4- met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116951 BindingDB Entry DOI: 10.7270/Q2HX1HM7 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-AMP-activated protein kinase catalytic subunit alpha-2 (Homo sapiens (Human)) | BDBM327994 (N-(1-(4- cyanobenzyl)piperidin-4- yl)-5-(4-(4- met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <500 | n/a | n/a | n/a | n/a |
Rigel Pharmaceuticals, Inc. US Patent | Assay Description Compounds were assayed for their ability to activate AMPK using an enzyme-linked immunosorbent assay. Reagents and procedures for measuring AMPK acti... | US Patent US10377742 (2019) BindingDB Entry DOI: 10.7270/Q2RV0R22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||