

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM347313 2-(4-(4-Ethoxy-6-oxo-1,6-dihydropyridin-3-yl)-2-fluorophenyl)-N-(4-(3-hydroxy-2,2-dimethylpropyl)-3-(trifluoromethyl)phenyl)acetamide::US9789100, Example 3
SMILES: CCOc1cc(=O)[nH]cc1-c1ccc(CC(=O)Nc2ccc(CC(C)(C)CO)c(c2)C(F)(F)F)c(F)c1
InChI Key: InChIKey=PCNMRGUJZFWHPU-UHFFFAOYSA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proto-oncogene tyrosine-protein kinase receptor Ret [658-1114] (Homo sapiens (Human)) | BDBM347313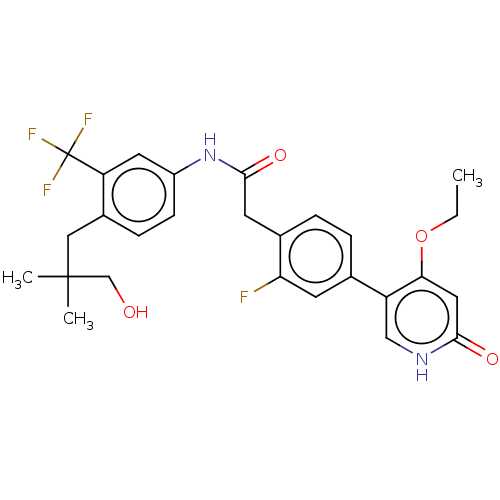 (2-(4-(4-Ethoxy-6-oxo-1,6-dihydropyridin-3-yl)-2-fl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE INTELLECTUAL PROPERTY DEVELOPMENT LIMITED US Patent | Assay Description Human RET kinase cytoplasmic domain (amino acids 658-1114 of accession number NP_000314.1) was expressed as an N-terminal GST-fusion protein using a ... | US Patent US9789100 (2017) BindingDB Entry DOI: 10.7270/Q2445PMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret [658-1114] (Homo sapiens (Human)) | BDBM347313 (2-(4-(4-Ethoxy-6-oxo-1,6-dihydropyridin-3-yl)-2-fl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE INTELLECTUAL PROPERTY DEVELOPMENT LIMITED US Patent | Assay Description Human RET kinase cytoplasmic domain (amino acids 658-1114 of accession number NP_000314.1) was expressed as an N-terminal GST-fusion protein using a ... | US Patent US9789100 (2017) BindingDB Entry DOI: 10.7270/Q2445PMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM347313 (2-(4-(4-Ethoxy-6-oxo-1,6-dihydropyridin-3-yl)-2-fl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human RET using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 1 hr by HT... | ACS Med Chem Lett 9: 623-628 (2018) Article DOI: 10.1021/acsmedchemlett.8b00035 BindingDB Entry DOI: 10.7270/Q2TB19GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM347313 (2-(4-(4-Ethoxy-6-oxo-1,6-dihydropyridin-3-yl)-2-fl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of RET phosphorylation in human TT cells after 2 hrs by ELISA | ACS Med Chem Lett 9: 623-628 (2018) Article DOI: 10.1021/acsmedchemlett.8b00035 BindingDB Entry DOI: 10.7270/Q2TB19GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM347313 (2-(4-(4-Ethoxy-6-oxo-1,6-dihydropyridin-3-yl)-2-fl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human KDR using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by... | ACS Med Chem Lett 9: 623-628 (2018) Article DOI: 10.1021/acsmedchemlett.8b00035 BindingDB Entry DOI: 10.7270/Q2TB19GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret [658-1114] (Homo sapiens (Human)) | BDBM347313 (2-(4-(4-Ethoxy-6-oxo-1,6-dihydropyridin-3-yl)-2-fl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE INTELLECTUAL PROPERTY DEVELOPMENT LIMITED US Patent | Assay Description Human RET kinase cytoplasmic domain (amino acids 658-1114 of accession number NP_000314.1) was expressed as an N-terminal GST-fusion protein using a ... | US Patent US9789100 (2017) BindingDB Entry DOI: 10.7270/Q2445PMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||