

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM370419 2-(5H-imidazo[5,1-a]isoindol-5-yl)ethanol::US10233190, Example 1254
SMILES: OCCC1c2ccccc2-c2cncn12
InChI Key: InChIKey=VQGLVXRSMONFQT-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indoleamine 2,3-dioxygenase (Homo sapiens (Human)) | BDBM370419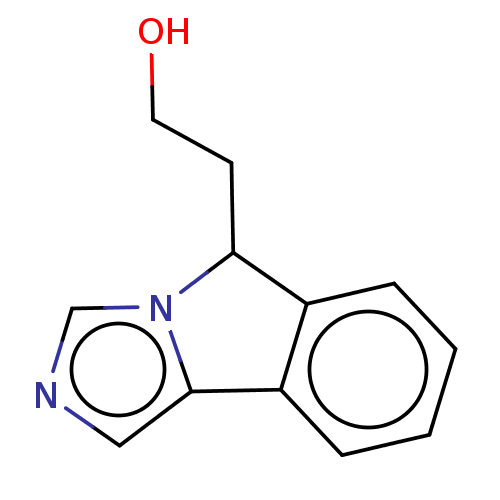 (2-(5H-imidazo[5,1-a]isoindol-5-yl)ethanol | US1023...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description The IC50 values for each compound were determined by testing the activity of IDO in a mixture containing 50 mM potassium phosphate buffer at pH 6.5; ... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q26H4KQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM370419 (2-(5H-imidazo[5,1-a]isoindol-5-yl)ethanol | US1023...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human TDO using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins... | ACS Med Chem Lett 11: 541-549 (2020) Article DOI: 10.1021/acsmedchemlett.0c00004 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase (Homo sapiens (Human)) | BDBM370419 (2-(5H-imidazo[5,1-a]isoindol-5-yl)ethanol | US1023...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a |
NewLink Genetics Corporation Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 expressed in T-REx-293 cells assessed as reduction in kynurenine level measured after 16 hrs | J Med Chem 62: 6705-6733 (2019) Article DOI: 10.1021/acs.jmedchem.9b00662 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase (Homo sapiens (Human)) | BDBM370419 (2-(5H-imidazo[5,1-a]isoindol-5-yl)ethanol | US1023...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NewLink Genetics Corporation Curated by ChEMBL | Assay Description Inhibition of purified human IDO1 using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins b... | J Med Chem 62: 6705-6733 (2019) Article DOI: 10.1021/acs.jmedchem.9b00662 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||