

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM392144 US10301272, Example 15/6
SMILES: CC(C)(C)NS(=O)(=O)c1ccc(-c2sc(nc2CC2CCCCC2)C(=O)N2CC3(C2)CS(=O)(=O)C3)c(Cl)c1Cl
InChI Key: InChIKey=UEXPQLLLMKWEBF-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isoform 2 of Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM392144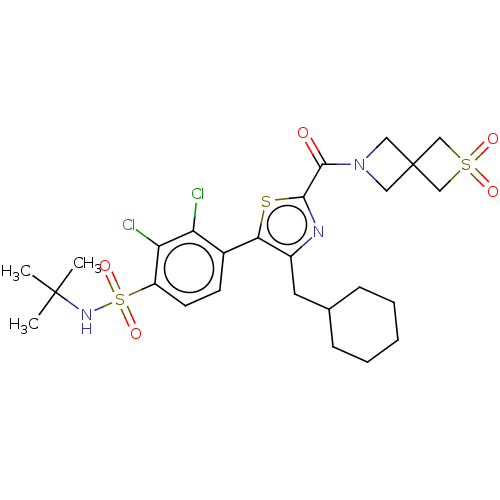 (US10301272, Example 15/6) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 50.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Determination of a ligand mediated Gal4 promoter driven transactivation to quantify ligand binding to RORγ was performed as follows: DNA encodin... | J Med Chem 52: 1814-27 (2009) BindingDB Entry DOI: 10.7270/Q2S184TJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 2 of Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM392144 (US10301272, Example 15/6) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Cells were incubated for additional 16 h before firefly (FF) luciferase activities were measured sequentially in the same cell extract using a Dual-L... | J Med Chem 52: 1814-27 (2009) BindingDB Entry DOI: 10.7270/Q2S184TJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 2 of Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM392144 (US10301272, Example 15/6) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Cells were incubated for additional 16 h before renilla (REN) luciferase activities were measured sequentially in the same cell extract using a Dual-... | J Med Chem 52: 1814-27 (2009) BindingDB Entry DOI: 10.7270/Q2S184TJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (RORC) (Homo sapiens (Human)) | BDBM392144 (US10301272, Example 15/6) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 35 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inverse agonist activity at RORgammat in human whole blood assessed as reduction in IL17A production | Bioorg Med Chem Lett 28: 1446-1455 (2018) Article DOI: 10.1016/j.bmcl.2018.03.093 BindingDB Entry DOI: 10.7270/Q27W6FPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-beta (Homo sapiens (Human)) | BDBM392144 (US10301272, Example 15/6) | PDB MMDB Reactome pathway UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Binding affinity to recombinant human RORbeta LBD (201 to 452 residues) expressed in Escherichia coli BL21 (DE3) GOLD by 1,8-ANS dye-based thermofluo... | Bioorg Med Chem Lett 28: 1446-1455 (2018) Article DOI: 10.1016/j.bmcl.2018.03.093 BindingDB Entry DOI: 10.7270/Q27W6FPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (RORC) (Homo sapiens (Human)) | BDBM392144 (US10301272, Example 15/6) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inverse agonist activity at RORgammat in human CD4 positive T cells assessed as inhibition of IL17A production in human Th17 cells after 1 hr | Bioorg Med Chem Lett 28: 1446-1455 (2018) Article DOI: 10.1016/j.bmcl.2018.03.093 BindingDB Entry DOI: 10.7270/Q27W6FPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (RORC) (Homo sapiens (Human)) | BDBM392144 (US10301272, Example 15/6) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inverse agonist activity at human GAL4 DBD-fused RORgammat LBD (237 to 497 residues) expressed in HEK293T cells assessed as reduction in ROR mediated... | Bioorg Med Chem Lett 28: 1446-1455 (2018) Article DOI: 10.1016/j.bmcl.2018.03.093 BindingDB Entry DOI: 10.7270/Q27W6FPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (RORC) (Homo sapiens (Human)) | BDBM392144 (US10301272, Example 15/6) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Binding affinity to recombinant human RORgamma LBD (237 to 497 residues) expressed in Escherichia coli BL21 (DE3) GOLD by 1,8-ANS dye-based thermoflu... | Bioorg Med Chem Lett 28: 1446-1455 (2018) Article DOI: 10.1016/j.bmcl.2018.03.093 BindingDB Entry DOI: 10.7270/Q27W6FPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||