

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CCN(C)C(=O)N1CCC(CC1)c1cc(-c2ccc(NC(=O)c3cc4C(=O)CCCc4n(-c4ccc(F)cc4)c3=O)cc2F)c2c(N)ncnn12
InChI Key: InChIKey=DUXXBRYGDLQJQM-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM396996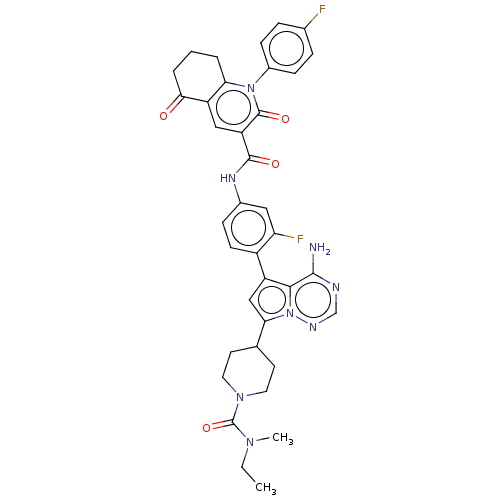 (US10442810, Example 58 | US10844069, Example 58 | ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Autophosphorylation of Axl was carried out by incubating the recombinant Axl protein (Life Technologies, PV4275) in buffer containing 50 mM Tris, pH7... | J Med Chem 52: 3576-85 (2009) BindingDB Entry DOI: 10.7270/Q2FB5593 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM396996 (US10442810, Example 58 | US10844069, Example 58 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Tyro3, and Mer:The kinase assay buffer contained 50 mM HEPES, pH7.5, 10 mM MgCl2, 1 mM EGTA, 0.01% NP-40 and 2 mM DTT. 0.1 ul test compounds dissolve... | J Med Chem 52: 3576-85 (2009) BindingDB Entry DOI: 10.7270/Q2FB5593 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM396996 (US10442810, Example 58 | US10844069, Example 58 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Tyro3, and Mer:The kinase assay buffer contained 50 mM HEPES, pH7.5, 10 mM MgCl2, 1 mM EGTA, 0.01% NP-40 and 2 mM DTT. 0.1 ul test compounds dissolve... | J Med Chem 52: 3576-85 (2009) BindingDB Entry DOI: 10.7270/Q2FB5593 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM396996 (US10442810, Example 58 | US10844069, Example 58 | ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description The kinase assay buffer contained 50 mM HEPES, pH7.5, 10 mM MgCl2, 1 mM EGTA, 0.01% NP-40 and 2 mM DTT. 0.1 ul test compounds dissolved in DMSO were ... | US Patent US10442810 (2019) BindingDB Entry DOI: 10.7270/Q2NG4T17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM396996 (US10442810, Example 58 | US10844069, Example 58 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description The kinase assay buffer contained 50 mM HEPES, pH7.5, 10 mM MgCl2, 1 mM EGTA, 0.01% NP-40 and 2 mM DTT. 0.1 ul test compounds dissolved in DMSO were ... | US Patent US10844069 (2020) BindingDB Entry DOI: 10.7270/Q2CZ3B73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM396996 (US10442810, Example 58 | US10844069, Example 58 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description The kinase assay buffer contained 50 mM HEPES, pH7.5, 10 mM MgCl2, 1 mM EGTA, 0.01% NP-40 and 2 mM DTT. 0.1 ul test compounds dissolved in DMSO were ... | US Patent US10442810 (2019) BindingDB Entry DOI: 10.7270/Q2NG4T17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM396996 (US10442810, Example 58 | US10844069, Example 58 | ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description The kinase assay buffer contained 50 mM HEPES, pH7.5, 10 mM MgCl2, 1 mM EGTA, 0.01% NP-40 and 2 mM DTT. 0.1 ul test compounds dissolved in DMSO were ... | US Patent US10844069 (2020) BindingDB Entry DOI: 10.7270/Q2CZ3B73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM396996 (US10442810, Example 58 | US10844069, Example 58 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description The kinase assay buffer contained 50 mM HEPES, pH7.5, 10 mM MgCl2, 1 mM EGTA, 0.01% NP-40 and 2 mM DTT. 0.1 ul test compounds dissolved in DMSO were ... | US Patent US10844069 (2020) BindingDB Entry DOI: 10.7270/Q2CZ3B73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM396996 (US10442810, Example 58 | US10844069, Example 58 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description The kinase assay buffer contained 50 mM HEPES, pH7.5, 10 mM MgCl2, 1 mM EGTA, 0.01% NP-40 and 2 mM DTT. 0.1 ul test compounds dissolved in DMSO were ... | US Patent US10442810 (2019) BindingDB Entry DOI: 10.7270/Q2NG4T17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||