

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM416666 (2S)-2-Methoxy-2-[3-methoxy-5-(trifluoromethoxy)phenyl]-N-[5-[[(3R)-1-pyridazin-3-ylpyrrolidin-3-yl]amino]-1,3,4-thiadiazol-2-yl]acetamide and (2R)-2-methoxy-2-[3-methoxy-5-(trifluoromethoxy)phenyl]-N-[5-[[(3R)-1-pyridazin-3-ylpyrrolidin-3-yl]amino]-1,3,4-thiadiazol-2-yl]acetamide::US10323028, Example 11(a)::US10981904, Example 11(b)
SMILES: CO[C@H](C(=O)Nc1nnc(N[C@@H]2CCN(C2)c2cccnn2)s1)c1cc(OC)cc(OC(F)(F)F)c1
InChI Key: InChIKey=ZFCXIZUETUNJKI-DYVFJYSZSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM416666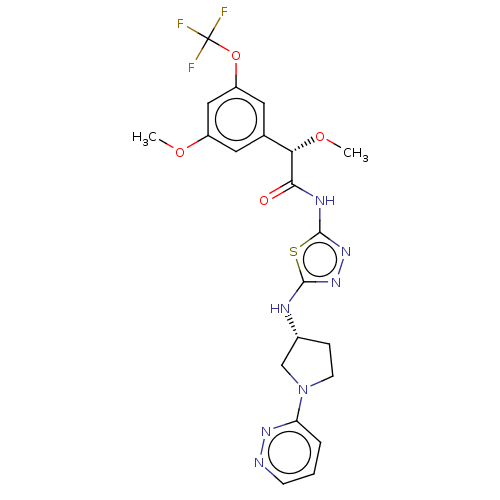 ((2S)-2-Methoxy-2-[3-methoxy-5-(trifluoromethoxy)ph...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.35 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description A Glutamate Oxidase/AmplexRed coupled assay was used to measure the ability of compounds to bind to and inhibit the activity of GLS1 in vitro. 6His t... | US Patent US10323028 (2019) BindingDB Entry DOI: 10.7270/Q20R9RTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (aa 63-669) (Homo sapiens (Human)) | BDBM416666 ((2S)-2-Methoxy-2-[3-methoxy-5-(trifluoromethoxy)ph...) | PDB GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.11 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Limited US Patent | Assay Description A Glutamate Oxidase/AmplexRed coupled assay was used to measure the ability of compounds to bind to and inhibit the activity of GLS1 in vitro. 6His t... | US Patent US10981904 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM416666 ((2S)-2-Methoxy-2-[3-methoxy-5-(trifluoromethoxy)ph...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human KGA (63 to 669 residues) preincubated for 15 mins using 50 mM glutamine as substrate by resorufin dye based assay | J Med Chem 62: 46-59 (2019) Article DOI: 10.1021/acs.jmedchem.8b00327 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||