

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CCCCNNC(=O)c1ccc(cc1)-c1cc(C)cc(F)c1
InChI Key: InChIKey=RVPBGXMIWLAEPK-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM468612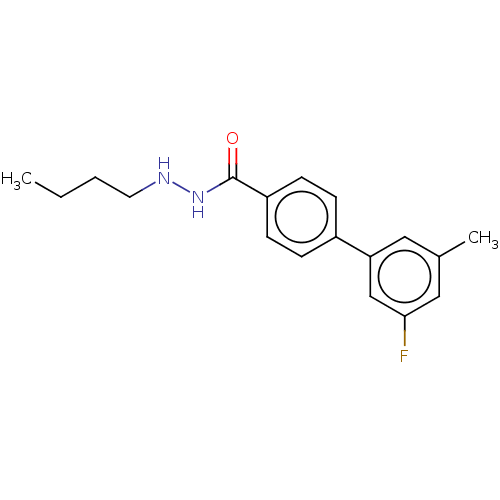 (US10807944, Compound SR-4372 | US11731934, Compoun...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Research Foundation, Inc.; The Scripps Research Institute US Patent | Assay Description These SAR data indicate that a tripartite structure of this scaffold with a central —C(O)—NH—NH— unit flanked by a phenyl group and a short aliphatic... | US Patent US10807944 (2020) BindingDB Entry DOI: 10.7270/Q2M048J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM468612 (US10807944, Compound SR-4372 | US11731934, Compoun...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Research Foundation, Inc.; The Scripps Research Institute US Patent | Assay Description These SAR data indicate that a tripartite structure of this scaffold with a central —C(O)—NH—NH— unit flanked by a phenyl group and a short aliphatic... | US Patent US10807944 (2020) BindingDB Entry DOI: 10.7270/Q2M048J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM468612 (US10807944, Compound SR-4372 | US11731934, Compoun...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Research Foundation, Inc.; The Scripps Research Institute US Patent | Assay Description These SAR data indicate that a tripartite structure of this scaffold with a central —C(O)—NH—NH— unit flanked by a phenyl group and a short aliphatic... | US Patent US10807944 (2020) BindingDB Entry DOI: 10.7270/Q2M048J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM468612 (US10807944, Compound SR-4372 | US11731934, Compoun...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Research Foundation, Inc.; The Scripps Research Institute US Patent | Assay Description These SAR data indicate that a tripartite structure of this scaffold with a central —C(O)—NH—NH— unit flanked by a phenyl group and a short aliphatic... | US Patent US10807944 (2020) BindingDB Entry DOI: 10.7270/Q2M048J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM468612 (US10807944, Compound SR-4372 | US11731934, Compoun...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2445RM9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM468612 (US10807944, Compound SR-4372 | US11731934, Compoun...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2445RM9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM468612 (US10807944, Compound SR-4372 | US11731934, Compoun...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2445RM9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM468612 (US10807944, Compound SR-4372 | US11731934, Compoun...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2445RM9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM468612 (US10807944, Compound SR-4372 | US11731934, Compoun...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2445RM9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM468612 (US10807944, Compound SR-4372 | US11731934, Compoun...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Research Foundation, Inc.; The Scripps Research Institute US Patent | Assay Description These SAR data indicate that a tripartite structure of this scaffold with a central —C(O)—NH—NH— unit flanked by a phenyl group and a short aliphatic... | US Patent US10807944 (2020) BindingDB Entry DOI: 10.7270/Q2M048J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||