

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: COc1ccc(cc1Cl)S(=O)(=O)Nc1cccc(-c2cn(C3CCN(C)CC3)c3ncnc(N)c23)c1F
InChI Key: InChIKey=CKXLOJPROMTISJ-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eukaryotic translation initiation factor 2-alpha kinase 3 [540-1115] (Homo sapiens (Human)) | BDBM482164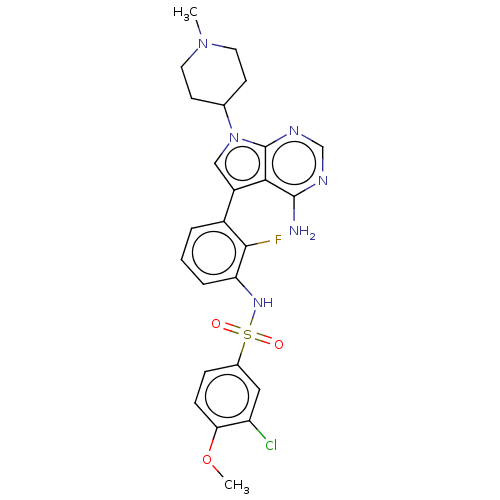 (N-{3-[4-Amino-7-(1-methyl-piperidin-4-yl)-7H-pyrro...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The biochemical activity of compounds was determined by incubation with PERK recombinant enzyme (cytoplasmic domain corresponding to residues 540-111... | Citation and Details BindingDB Entry DOI: 10.7270/Q2C2518M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 (Homo sapiens (Human)) | BDBM482164 (N-{3-[4-Amino-7-(1-methyl-piperidin-4-yl)-7H-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <16 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Compounds were 3-fold serially diluted in order to obtain from 3.333 to 0.000169 microM final concentration, then incubated for 60 minutes at room te... | US Patent US10918642 (2021) BindingDB Entry DOI: 10.7270/Q24Q7Z3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||