

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM48804 3-(phenylmethyl)-2-[(E)-2-(3-pyridinyl)ethenyl]-4-quinazolinone::3-(phenylmethyl)-2-[(E)-2-pyridin-3-ylethenyl]quinazolin-4-one::3-benzyl-2-[(E)-2-(3-pyridyl)vinyl]quinazolin-4-one::3-benzyl-2-[(E)-2-pyridin-3-ylethenyl]quinazolin-4-one::MLS000709026::SMR000289793::cid_5738263
SMILES: O=c1n(Cc2ccccc2)c(\C=C\c2cccnc2)nc2ccccc12
InChI Key: InChIKey=GEKDQXSPTHHANP-OUKQBFOZSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| eukaryotic translation initiation factor 4 gamma, 1 isoform 4 (Homo sapiens (Human)) | BDBM48804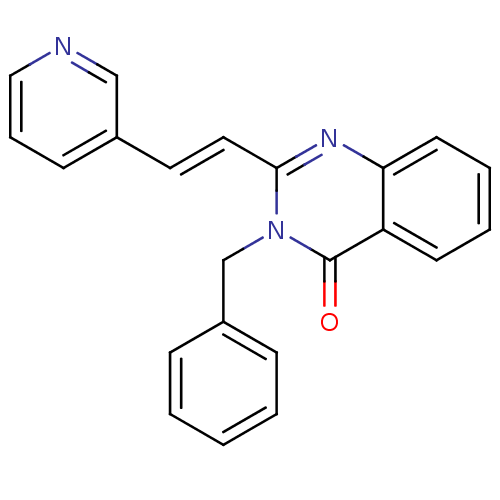 (3-(phenylmethyl)-2-[(E)-2-(3-pyridinyl)ethenyl]-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 5.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Molecular Libraries Screening Center Curated by PubChem BioAssay | Assay Description Dose Response Confirmation for Small Molecule Inhibitors of Eukaryotic Translation Initiation NIH Molecular Libraries Screening Centers Network [MLSC... | PubChem Bioassay (2009) BindingDB Entry DOI: 10.7270/Q2RN3686 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein RAD51 homolog 1 (Homo sapiens (Human)) | BDBM48804 (3-(phenylmethyl)-2-[(E)-2-(3-pyridinyl)ethenyl]-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 4.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
PCMD Curated by PubChem BioAssay | Assay Description Project Title: A screen for modulators of human Rad51, a key DNA repair protein Application Number: MH084119 Assay Submitter: Dr. Alex Mazin Submitte... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2VD6WW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein RAD51 homolog 1 (Homo sapiens (Human)) | BDBM48804 (3-(phenylmethyl)-2-[(E)-2-(3-pyridinyl)ethenyl]-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
PCMD Curated by PubChem BioAssay | Assay Description Project Title: A screen for modulators of human Rad51, a key DNA repair protein Application Number: MH084119 Assay Submitter: Dr. Alex Mazin Submitte... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2QN6558 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| putative hexokinase HKDC1 (Homo sapiens (Human)) | BDBM48804 (3-(phenylmethyl)-2-[(E)-2-(3-pyridinyl)ethenyl]-4-...) | GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2W094D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| putative hexokinase HKDC1 (Homo sapiens (Human)) | BDBM48804 (3-(phenylmethyl)-2-[(E)-2-(3-pyridinyl)ethenyl]-4-...) | GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q23F4N3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein RAD51 homolog 1 (Homo sapiens (Human)) | BDBM48804 (3-(phenylmethyl)-2-[(E)-2-(3-pyridinyl)ethenyl]-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore Curated by ChEMBL | Assay Description Inhibition of human RAD51 | Bioorg Med Chem Lett 29: 2286-2289 (2019) Article DOI: 10.1016/j.bmcl.2019.06.024 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein RAD51 homolog 1 (Homo sapiens (Human)) | BDBM48804 (3-(phenylmethyl)-2-[(E)-2-(3-pyridinyl)ethenyl]-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a |
Drexel University College of Medicine Curated by ChEMBL | Assay Description Binding affinity to human RAD51 in absence of ATP by SPR method | J Med Chem 55: 3011-20 (2012) Article DOI: 10.1021/jm201173g BindingDB Entry DOI: 10.7270/Q2028SKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell cycle checkpoint protein RAD1 (Homo sapiens (Human)) | BDBM48804 (3-(phenylmethyl)-2-[(E)-2-(3-pyridinyl)ethenyl]-4-...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a |
Drexel University College of Medicine Curated by ChEMBL | Assay Description Inhibition of RAD1 (unknown origin) binding to ssDNA | Bioorg Med Chem Lett 24: 3006-13 (2014) Article DOI: 10.1016/j.bmcl.2014.04.088 BindingDB Entry DOI: 10.7270/Q2NV9KW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell cycle checkpoint protein RAD1 (Homo sapiens (Human)) | BDBM48804 (3-(phenylmethyl)-2-[(E)-2-(3-pyridinyl)ethenyl]-4-...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a |
Drexel University College of Medicine Curated by ChEMBL | Assay Description Binding affinity to RAD1 (unknown origin) by surface plasmon resonance method | Bioorg Med Chem Lett 24: 3006-13 (2014) Article DOI: 10.1016/j.bmcl.2014.04.088 BindingDB Entry DOI: 10.7270/Q2NV9KW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein RAD51 homolog 1 (Homo sapiens (Human)) | BDBM48804 (3-(phenylmethyl)-2-[(E)-2-(3-pyridinyl)ethenyl]-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Drexel University College of Medicine Curated by ChEMBL | Assay Description Inhibition of human RAD51-mediated DNA branch migration using [32P]-labeled 5'-joint molecules after 8 hrs by branch migration assay | J Med Chem 55: 3011-20 (2012) Article DOI: 10.1021/jm201173g BindingDB Entry DOI: 10.7270/Q2028SKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein RAD51 homolog 1 (Homo sapiens (Human)) | BDBM48804 (3-(phenylmethyl)-2-[(E)-2-(3-pyridinyl)ethenyl]-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Drexel University College of Medicine Curated by ChEMBL | Assay Description Inhibition of human RAD51-mediated DNA strand exchange at using pBSK (+) gapped and linear dsDNA substrates by joint molecule formation assay | J Med Chem 55: 3011-20 (2012) Article DOI: 10.1021/jm201173g BindingDB Entry DOI: 10.7270/Q2028SKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||