 Found 7 hits for monomerid = 50010464
Found 7 hits for monomerid = 50010464 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50010464
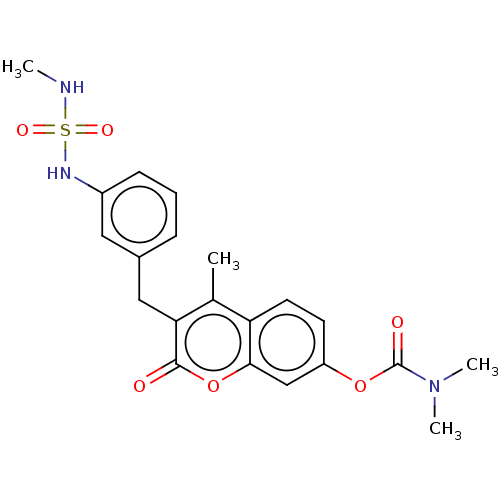
(CHEMBL3092189)Show SMILES CNS(=O)(=O)Nc1cccc(Cc2c(C)c3ccc(OC(=O)N(C)C)cc3oc2=O)c1 Show InChI InChI=1S/C21H23N3O6S/c1-13-17-9-8-16(29-21(26)24(3)4)12-19(17)30-20(25)18(13)11-14-6-5-7-15(10-14)23-31(27,28)22-2/h5-10,12,22-23H,11H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using diclofenac as substrate measured after 30 mins preincubation by LC-MS/MS analysis in absence of ... |
ACS Med Chem Lett 5: 309-14 (2014)
Article DOI: 10.1021/ml400379x
BindingDB Entry DOI: 10.7270/Q27W6DQD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50010464

(CHEMBL3092189)Show SMILES CNS(=O)(=O)Nc1cccc(Cc2c(C)c3ccc(OC(=O)N(C)C)cc3oc2=O)c1 Show InChI InChI=1S/C21H23N3O6S/c1-13-17-9-8-16(29-21(26)24(3)4)12-19(17)30-20(25)18(13)11-14-6-5-7-15(10-14)23-31(27,28)22-2/h5-10,12,22-23H,11H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using diclofenac as substrate measured after 30 mins preincubation by LC-MS/MS analysis in presence of... |
ACS Med Chem Lett 5: 309-14 (2014)
Article DOI: 10.1021/ml400379x
BindingDB Entry DOI: 10.7270/Q27W6DQD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50010464

(CHEMBL3092189)Show SMILES CNS(=O)(=O)Nc1cccc(Cc2c(C)c3ccc(OC(=O)N(C)C)cc3oc2=O)c1 Show InChI InChI=1S/C21H23N3O6S/c1-13-17-9-8-16(29-21(26)24(3)4)12-19(17)30-20(25)18(13)11-14-6-5-7-15(10-14)23-31(27,28)22-2/h5-10,12,22-23H,11H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate measured after 30 mins preincubation by LC-MS/MS analysis in absence of N... |
ACS Med Chem Lett 5: 309-14 (2014)
Article DOI: 10.1021/ml400379x
BindingDB Entry DOI: 10.7270/Q27W6DQD |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1/Mitogen-activated protein kinase 1/RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50010464

(CHEMBL3092189)Show SMILES CNS(=O)(=O)Nc1cccc(Cc2c(C)c3ccc(OC(=O)N(C)C)cc3oc2=O)c1 Show InChI InChI=1S/C21H23N3O6S/c1-13-17-9-8-16(29-21(26)24(3)4)12-19(17)30-20(25)18(13)11-14-6-5-7-15(10-14)23-31(27,28)22-2/h5-10,12,22-23H,11H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of MEK1 (unknown origin) assessed as phosphorylation of Erk2 preincubated for 30 mins followed by FAM-Erktide addition measured after 60 m... |
ACS Med Chem Lett 4: 1059-63 (2013)
Article DOI: 10.1021/ml4002419
BindingDB Entry DOI: 10.7270/Q20K2CJZ |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50010464

(CHEMBL3092189)Show SMILES CNS(=O)(=O)Nc1cccc(Cc2c(C)c3ccc(OC(=O)N(C)C)cc3oc2=O)c1 Show InChI InChI=1S/C21H23N3O6S/c1-13-17-9-8-16(29-21(26)24(3)4)12-19(17)30-20(25)18(13)11-14-6-5-7-15(10-14)23-31(27,28)22-2/h5-10,12,22-23H,11H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of truncated active C-Raf (unknown origin) assessed as MEK1 phosphorylation using inactive MEK1 K97R as substrate after 45 mins |
ACS Med Chem Lett 5: 309-14 (2014)
Article DOI: 10.1021/ml400379x
BindingDB Entry DOI: 10.7270/Q27W6DQD |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50010464

(CHEMBL3092189)Show SMILES CNS(=O)(=O)Nc1cccc(Cc2c(C)c3ccc(OC(=O)N(C)C)cc3oc2=O)c1 Show InChI InChI=1S/C21H23N3O6S/c1-13-17-9-8-16(29-21(26)24(3)4)12-19(17)30-20(25)18(13)11-14-6-5-7-15(10-14)23-31(27,28)22-2/h5-10,12,22-23H,11H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of C-Raf (unknown origin) assessed as phosphorylation of MEK1 after 45 mins by TR-FRET assay |
ACS Med Chem Lett 4: 1059-63 (2013)
Article DOI: 10.1021/ml4002419
BindingDB Entry DOI: 10.7270/Q20K2CJZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50010464

(CHEMBL3092189)Show SMILES CNS(=O)(=O)Nc1cccc(Cc2c(C)c3ccc(OC(=O)N(C)C)cc3oc2=O)c1 Show InChI InChI=1S/C21H23N3O6S/c1-13-17-9-8-16(29-21(26)24(3)4)12-19(17)30-20(25)18(13)11-14-6-5-7-15(10-14)23-31(27,28)22-2/h5-10,12,22-23H,11H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate measured after 30 mins preincubation by LC-MS/MS analysis in presence of ... |
ACS Med Chem Lett 5: 309-14 (2014)
Article DOI: 10.1021/ml400379x
BindingDB Entry DOI: 10.7270/Q27W6DQD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data