

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50019420 CHEMBL3290104
SMILES: COc1cccc(OC)c1-c1cc(nn1-c1ccc(F)cc1)C(=O)N[C@@H](C1CCCCC1)C(O)=O
InChI Key: InChIKey=WXWDASPOKBCBCE-DEOSSOPVSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neurotensin receptor 2 (Rattus norvegicus) | BDBM50019420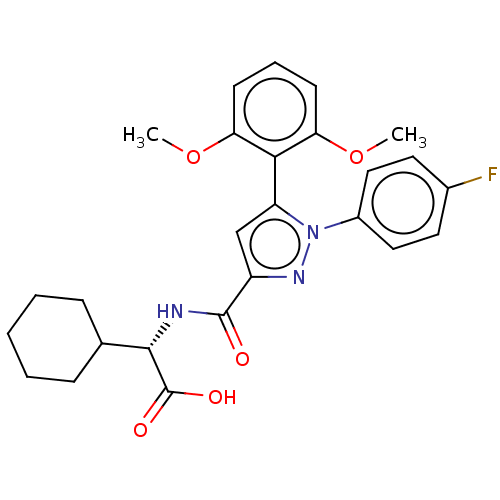 (CHEMBL3290104) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 622 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [125I]NT at rat NTS2 overexpressed in CHOK1 cells after 30 mins by gamma counting | J Med Chem 57: 5318-32 (2014) Article DOI: 10.1021/jm5003843 BindingDB Entry DOI: 10.7270/Q2PR7XJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor 2 (Rattus norvegicus) | BDBM50019420 (CHEMBL3290104) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 271 | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Agonist activity at rat NTS2 stably expressed in CHOK1 cells assessed as induction of calcium release by FLIPR assay | J Med Chem 57: 5318-32 (2014) Article DOI: 10.1021/jm5003843 BindingDB Entry DOI: 10.7270/Q2PR7XJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||