

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50027109 2,15,17,29-tetrahydroxy-11-methoxy-3,7,12,14,16,18,22-heptamethyl-26-carbaldehyde O-benzhydryl-oxime-6,23-dioxo-8,30-dioxa-24-azatetracyclo[23.3.1.14,7.05,28]triaconta-1(28),2,4,9,19,21,25(29),26-octaen-13-yl acetate::CHEMBL407384
SMILES: COC1\C=C\OC2(C)Oc3c(C2=O)c2cc(\C=N\OC(c4ccccc4)c4ccccc4)c(NC(=O)\C(C)=C/C=C/C(C)C(O)C(C)C(O)C(C)C(OC(C)=O)C1C)c(O)c2c(O)c3C
InChI Key: InChIKey=MUPFBVFVAWNWMI-MFGIWUPUSA-N
Data: 2 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA polymerase beta (Mus musculus) | BDBM50027109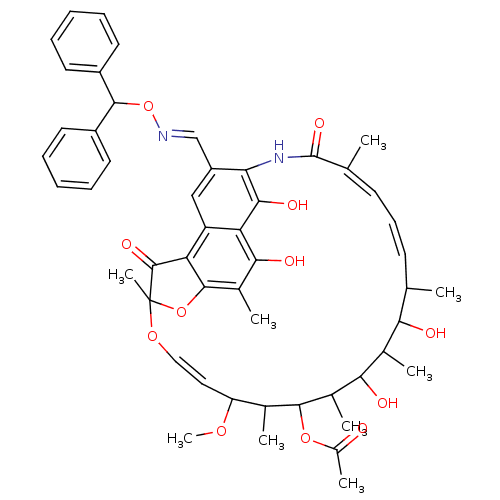 (2,15,17,29-tetrahydroxy-11-methoxy-3,7,12,14,16,18...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit mouse beta DNA polymerase | J Med Chem 23: 256-61 (1980) BindingDB Entry DOI: 10.7270/Q2XD10PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Simian sarcoma virus Pol protein (Woolly monkey sarcoma virus) | BDBM50027109 (2,15,17,29-tetrahydroxy-11-methoxy-3,7,12,14,16,18...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit simian sarcoma virus reverse transcriptase | J Med Chem 23: 256-61 (1980) BindingDB Entry DOI: 10.7270/Q2XD10PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||