

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50027791 6,11-Dimethyl-3-phenethyl-1,2,3,4,5,6-hexahydro-2,6-methano-benzo[d]azocin-8-ol(phenazocine)::CHEMBL46399::PHENAZOCINE
SMILES: CC1C2Cc3ccc(O)cc3C1(C)CCN2CCc1ccccc1
InChI Key: InChIKey=ZQHYKVKNPWDQSL-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50027791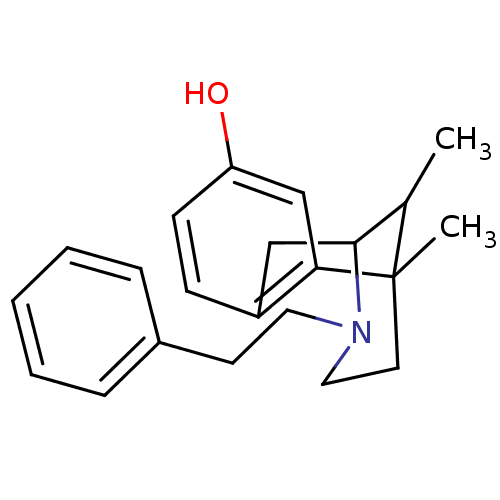 (6,11-Dimethyl-3-phenethyl-1,2,3,4,5,6-hexahydro-2,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO form human mu opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 203-8 (2008) Article DOI: 10.1016/j.bmcl.2008.10.134 BindingDB Entry DOI: 10.7270/Q2RX9D1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50027791 (6,11-Dimethyl-3-phenethyl-1,2,3,4,5,6-hexahydro-2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Activity at human cloned kappa opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 19: 203-8 (2008) Article DOI: 10.1016/j.bmcl.2008.10.134 BindingDB Entry DOI: 10.7270/Q2RX9D1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50027791 (6,11-Dimethyl-3-phenethyl-1,2,3,4,5,6-hexahydro-2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]Naltrindole form human delta opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 203-8 (2008) Article DOI: 10.1016/j.bmcl.2008.10.134 BindingDB Entry DOI: 10.7270/Q2RX9D1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50027791 (6,11-Dimethyl-3-phenethyl-1,2,3,4,5,6-hexahydro-2,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Activity at human cloned mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 19: 203-8 (2008) Article DOI: 10.1016/j.bmcl.2008.10.134 BindingDB Entry DOI: 10.7270/Q2RX9D1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50027791 (6,11-Dimethyl-3-phenethyl-1,2,3,4,5,6-hexahydro-2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to induce 50% of maximal effect in mouse vas deferens expressing Opioid receptor delta 1 | J Med Chem 26: 1643-5 (1983) BindingDB Entry DOI: 10.7270/Q2N015J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50027791 (6,11-Dimethyl-3-phenethyl-1,2,3,4,5,6-hexahydro-2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to induce 50% of maximal effect in rabbit vas deferens expressing Opioid receptor kappa 1 | J Med Chem 26: 1643-5 (1983) BindingDB Entry DOI: 10.7270/Q2N015J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50027791 (6,11-Dimethyl-3-phenethyl-1,2,3,4,5,6-hexahydro-2,...) | UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to induce 50% of maximal effect in guinea pig ileum expressing Opioid receptor mu 1 | J Med Chem 26: 1643-5 (1983) BindingDB Entry DOI: 10.7270/Q2N015J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50027791 (6,11-Dimethyl-3-phenethyl-1,2,3,4,5,6-hexahydro-2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Activity at human cloned kappa opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 19: 203-8 (2008) Article DOI: 10.1016/j.bmcl.2008.10.134 BindingDB Entry DOI: 10.7270/Q2RX9D1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||