

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50028963 3-Phenyl-1H-naphtho[2,1-b]pyran-1-one::3-Phenyl-benzo[f]chromen-1-one::3-phenyl-1H-benzo[f]chromen-1-one::CHEMBL26260::beta -naphthoflavone::beta-naphthoflavone
SMILES: O=c1cc(oc2ccc3ccccc3c12)-c1ccccc1
InChI Key: InChIKey=OUGIDAPQYNCXRA-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50028963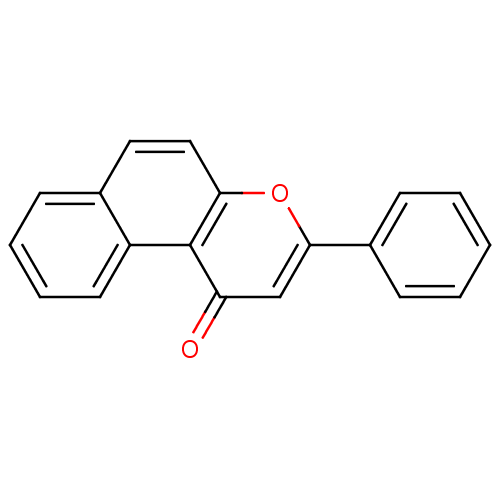 (3-Phenyl-1H-naphtho[2,1-b]pyran-1-one | 3-Phenyl-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Displacement of specific [3H]-PIA binding from adenosine A1 receptor in rat brain membranes. | J Med Chem 39: 781-8 (1996) Article DOI: 10.1021/jm950661k BindingDB Entry DOI: 10.7270/Q2M32TV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Homo sapiens (Human)) | BDBM50028963 (3-Phenyl-1H-naphtho[2,1-b]pyran-1-one | 3-Phenyl-b...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Agonist activity at aryl hydrocarbon receptor in human MCF7 cells after 24 hrs CYP1A1-dependent EROD assay | Bioorg Med Chem 18: 1194-203 (2010) Article DOI: 10.1016/j.bmc.2009.12.036 BindingDB Entry DOI: 10.7270/Q2GX4BNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Homo sapiens (Human)) | BDBM50028963 (3-Phenyl-1H-naphtho[2,1-b]pyran-1-one | 3-Phenyl-b...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Agonist activity at AhR in human MCF-7 cells assessed as increase of CYP1A1-dependent 7-ethoxyresorufin O-deethylase activity | J Med Chem 54: 1539-54 (2011) Article DOI: 10.1021/jm101356p BindingDB Entry DOI: 10.7270/Q27S7P30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Homo sapiens (Human)) | BDBM50028963 (3-Phenyl-1H-naphtho[2,1-b]pyran-1-one | 3-Phenyl-b...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a |
University of Perugia Curated by ChEMBL | Assay Description Agonist activity at AhR (unknown origin) | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111842 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-binding cassette sub-family G member 2 (Homo sapiens (Human)) | BDBM50028963 (3-Phenyl-1H-naphtho[2,1-b]pyran-1-one | 3-Phenyl-b...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human BCRP expressed in MDCK2 cells assessed as accumulation of pheophorbide-A preincubated for 30 mins before pheophorbide-A addition ... | Eur J Med Chem 67: 115-26 (2013) Article DOI: 10.1016/j.ejmech.2013.06.035 BindingDB Entry DOI: 10.7270/Q2W66N6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B6 (Homo sapiens (Human)) | BDBM50028963 (3-Phenyl-1H-naphtho[2,1-b]pyran-1-one | 3-Phenyl-b...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Xavier University of Louisiana Curated by ChEMBL | Assay Description Inhibition of CYP2B1 (unknown origin)-mediated depentylation of resorufin pentyl ether after 5 mins by spectrofluorimetric analysis | J Med Chem 56: 4082-92 (2013) Article DOI: 10.1021/jm4003654 BindingDB Entry DOI: 10.7270/Q2CN758W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A (Homo sapiens (Human)) | BDBM50028963 (3-Phenyl-1H-naphtho[2,1-b]pyran-1-one | 3-Phenyl-b...) | PDB MMDB Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Xavier University of Louisiana Curated by ChEMBL | Assay Description Inhibition of CYP1A2 (unknown origin)-mediated demethylation of resorufin methyl ether after 5 mins by spectrofluorimetric analysis | J Med Chem 56: 4082-92 (2013) Article DOI: 10.1021/jm4003654 BindingDB Entry DOI: 10.7270/Q2CN758W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50028963 (3-Phenyl-1H-naphtho[2,1-b]pyran-1-one | 3-Phenyl-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Xavier University of Louisiana Curated by ChEMBL | Assay Description Inhibition of CYP1A1 (unknown origin)-mediated deethylation of resorufin ethyl ether after 5 mins by spectrofluorimetric analysis | J Med Chem 56: 4082-92 (2013) Article DOI: 10.1021/jm4003654 BindingDB Entry DOI: 10.7270/Q2CN758W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM50028963 (3-Phenyl-1H-naphtho[2,1-b]pyran-1-one | 3-Phenyl-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Xavier University of Louisiana Curated by ChEMBL | Assay Description Inhibition of CYP2A6 (unknown origin)-mediated coumarin 7-hydroxylation after 5 mins by spectrofluorimetric analysis | J Med Chem 56: 4082-92 (2013) Article DOI: 10.1021/jm4003654 BindingDB Entry DOI: 10.7270/Q2CN758W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-binding cassette sub-family G member 2 (Homo sapiens (Human)) | BDBM50028963 (3-Phenyl-1H-naphtho[2,1-b]pyran-1-one | 3-Phenyl-b...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human BCRP expressed in MDCK2 cells assessed as accumulation of Hoechst 33342 preincubated for 30 mins before Hoechst 33342 addition me... | Eur J Med Chem 67: 115-26 (2013) Article DOI: 10.1016/j.ejmech.2013.06.035 BindingDB Entry DOI: 10.7270/Q2W66N6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||