

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50049400 (+/-) taxifolin::(+/-)-Dihydroquercetin::(+/-)-taxifolin::(2R,3R)-2-(3,4-Dihydroxy-phenyl)-3,5,7-trihydroxy-chroman-4-one::(2R,3R)-2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxychroman-4-one::2-(3,4-Dihydroxy-phenyl)-3,5,7-trihydroxy-chroman-4-one::2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxychroman-4-one::3,5,7,3',4'-pentahydroxyflavanone::CHEMBL337309::NSC-2801
SMILES: OC1C(=O)C(Oc2cc(O)cc(O)c12)c1ccc(O)c(O)c1
InChI Key: InChIKey=BYVWXQRJRYELNP-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50049400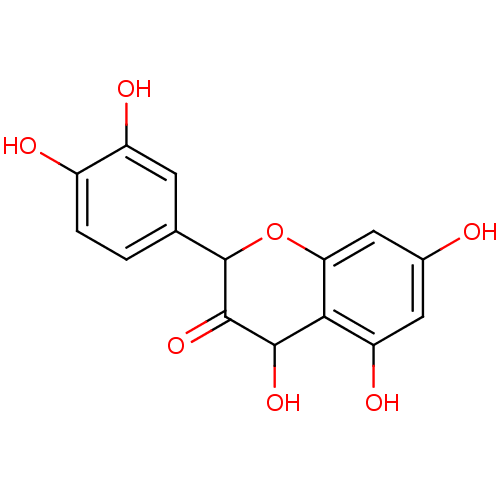 ((+/-) taxifolin | (+/-)-Dihydroquercetin | (+/-)-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 33 | -10.2 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Aristotle University of Thessaloniki Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins at room temperature/6 hrs at 4 deg C by stopped-flow CO2 hydration ass... | Bioorg Med Chem 23: 7219-25 (2015) BindingDB Entry DOI: 10.7270/Q2862J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50049400 ((+/-) taxifolin | (+/-)-Dihydroquercetin | (+/-)-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 493 | -8.60 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Aristotle University of Thessaloniki Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 7 preincubated for 15 mins at room temperature/6 hrs at 4 deg C by stopped-flow CO2 hydration assa... | Bioorg Med Chem 23: 7219-25 (2015) BindingDB Entry DOI: 10.7270/Q2862J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM50049400 ((+/-) taxifolin | (+/-)-Dihydroquercetin | (+/-)-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 9.10E+3 | -6.87 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Aristotle University of Thessaloniki Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 4 preincubated for 15 mins at room temperature/6 hrs at 4 deg C by stopped-flow CO2 hydration assa... | Bioorg Med Chem 23: 7219-25 (2015) BindingDB Entry DOI: 10.7270/Q2862J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50049400 ((+/-) taxifolin | (+/-)-Dihydroquercetin | (+/-)-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+4 | >-6.82 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Aristotle University of Thessaloniki Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 preincubated for 15 mins at room temperature/6 hrs at 4 deg C by stopped-flow CO2 hydration assa... | Bioorg Med Chem 23: 7219-25 (2015) BindingDB Entry DOI: 10.7270/Q2862J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50049400 ((+/-) taxifolin | (+/-)-Dihydroquercetin | (+/-)-t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+4 | >-6.82 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Aristotle University of Thessaloniki Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 1 preincubated for 15 mins at room temperature/6 hrs at 4 deg C by stopped-flow CO2 hydration assa... | Bioorg Med Chem 23: 7219-25 (2015) BindingDB Entry DOI: 10.7270/Q2862J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50049400 ((+/-) taxifolin | (+/-)-Dihydroquercetin | (+/-)-t...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Ability to displace [125I]-AB-MECA binding from adenosine A3 receptor. | J Med Chem 41: 46-52 (1998) Checked by Author Article DOI: 10.1021/jm970446z BindingDB Entry DOI: 10.7270/Q2NC62QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50049400 ((+/-) taxifolin | (+/-)-Dihydroquercetin | (+/-)-t...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Displacement of [125I]-AB-MECA binding to human Adenosine A3 receptor expressed in HEK-293 cells | J Med Chem 39: 781-8 (1996) Article DOI: 10.1021/jm950661k BindingDB Entry DOI: 10.7270/Q2M32TV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50049400 ((+/-) taxifolin | (+/-)-Dihydroquercetin | (+/-)-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant MMP9 catalytic domain incubated for 20 mins by fluorimetric assay | Bioorg Med Chem 20: 4164-71 (2012) Article DOI: 10.1016/j.bmc.2012.04.063 BindingDB Entry DOI: 10.7270/Q22808NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-12 (MMP12) (Homo sapiens (Human)) | BDBM50049400 ((+/-) taxifolin | (+/-)-Dihydroquercetin | (+/-)-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant MMP12 catalytic domain incubated for 20 mins by fluorimetric assay | Bioorg Med Chem 20: 4164-71 (2012) Article DOI: 10.1016/j.bmc.2012.04.063 BindingDB Entry DOI: 10.7270/Q22808NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, muscle form (Oryctolagus cuniculus (rabbit)) | BDBM50049400 ((+/-) taxifolin | (+/-)-Dihydroquercetin | (+/-)-t...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon Curated by ChEMBL | Assay Description Binding affinity to rabbit muscular GPb by NMR binding assay | J Med Chem 55: 1287-95 (2012) Article DOI: 10.1021/jm201439b BindingDB Entry DOI: 10.7270/Q21J9BS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, muscle form (Oryctolagus cuniculus (rabbit)) | BDBM50049400 ((+/-) taxifolin | (+/-)-Dihydroquercetin | (+/-)-t...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon Curated by ChEMBL | Assay Description Binding affinity to rabbit muscular GPa by NMR binding assay | J Med Chem 55: 1287-95 (2012) Article DOI: 10.1021/jm201439b BindingDB Entry DOI: 10.7270/Q21J9BS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50049400 ((+/-) taxifolin | (+/-)-Dihydroquercetin | (+/-)-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant MMP3 catalytic domain incubated for 20 mins by fluorimetric assay | Bioorg Med Chem 20: 4164-71 (2012) Article DOI: 10.1016/j.bmc.2012.04.063 BindingDB Entry DOI: 10.7270/Q22808NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50049400 ((+/-) taxifolin | (+/-)-Dihydroquercetin | (+/-)-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant MMP2 catalytic domain incubated for 20 mins by fluorimetric assay | Bioorg Med Chem 20: 4164-71 (2012) Article DOI: 10.1016/j.bmc.2012.04.063 BindingDB Entry DOI: 10.7270/Q22808NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50049400 ((+/-) taxifolin | (+/-)-Dihydroquercetin | (+/-)-t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant MMP13 catalytic domain incubated for 20 mins by fluorimetric assay | Bioorg Med Chem 20: 4164-71 (2012) Article DOI: 10.1016/j.bmc.2012.04.063 BindingDB Entry DOI: 10.7270/Q22808NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50049400 ((+/-) taxifolin | (+/-)-Dihydroquercetin | (+/-)-t...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase assessed as decrease in uric acid production by spectrophotometry | J Nat Prod 61: 71-6 (1998) Article DOI: 10.1021/np970237h BindingDB Entry DOI: 10.7270/Q29C6Z93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50049400 ((+/-) taxifolin | (+/-)-Dihydroquercetin | (+/-)-t...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Inhibition of Helicobacter pylori ATCC 43504 urease-mediated ammonia production preincubated for 1.5 hrs by indophenol method | Eur J Med Chem 63: 685-95 (2013) Article DOI: 10.1016/j.ejmech.2013.03.016 BindingDB Entry DOI: 10.7270/Q2D79FB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-glycoprotein (P-gp) (Mus musculus (Mouse)) | BDBM50049400 ((+/-) taxifolin | (+/-)-Dihydroquercetin | (+/-)-t...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | 3.74E+4 | n/a | n/a | n/a | n/a | n/a |
Universit£ Claude Bernard-Lyon 1 Curated by ChEMBL | Assay Description Compound was tested for the binding affinity towards recombinant NBD2 C-terminal cytotoxic nucleotide-binding domain of mouse P-Glycoprotein | Bioorg Med Chem Lett 10: 157-60 (2000) BindingDB Entry DOI: 10.7270/Q2DR2W1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lymphocyte differentiation antigen CD38 (Homo sapiens (Human)) | BDBM50049400 ((+/-) taxifolin | (+/-)-Dihydroquercetin | (+/-)-t...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 7200 CNRS-Universit£ de Strasbourg Curated by ChEMBL | Assay Description Inhibition of human CD38 using 20 uM 1, N6-etheno NAD+ as substrate by continuous fluorimetric method | Bioorg Med Chem Lett 21: 3939-42 (2011) Article DOI: 10.1016/j.bmcl.2011.05.022 BindingDB Entry DOI: 10.7270/Q2SF2WJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50049400 ((+/-) taxifolin | (+/-)-Dihydroquercetin | (+/-)-t...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dongguk University-Seoul Curated by ChEMBL | Assay Description Inhibition of recombinant FLT3 (unknown origin) by TR-FRET assay | Bioorg Med Chem Lett 23: 1768-70 (2013) Article DOI: 10.1016/j.bmcl.2013.01.049 BindingDB Entry DOI: 10.7270/Q2H70H54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||