

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50059084 CHEMBL3393171::US10308663, Thienopyridone (5)
SMILES: Nc1c[nH]c(=O)c2cc(sc12)-c1ccccc1
InChI Key: InChIKey=FBVOPOZVLBHOHR-UHFFFAOYSA-N
Data: 10 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein tyrosine phosphatase type IVA 1 (Homo sapiens (Human)) | BDBM50059084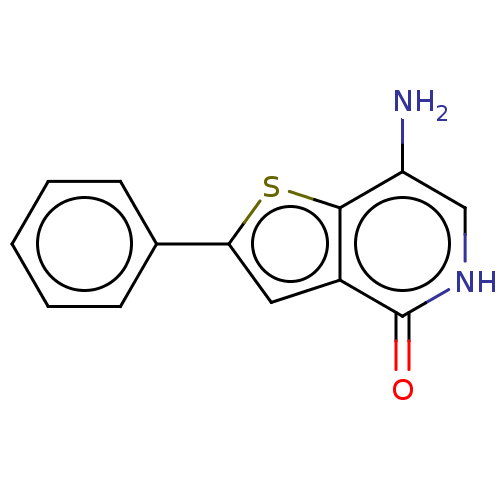 (CHEMBL3393171 | US10308663, Thienopyridone (5)) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 173 | n/a | n/a | n/a | n/a | n/a | n/a |
European Molecular Biology Laboratory Curated by ChEMBL | Assay Description Inhibition of full length human fusion His6 tagged PRL1 expressed in Escherichia coli using TAMRA-Thr-Ala-Asp-Ile-Tyr(PO3H2)-Glu-NH2 substrate by imm... | Eur J Med Chem 88: 89-100 (2014) Article DOI: 10.1016/j.ejmech.2014.08.060 BindingDB Entry DOI: 10.7270/Q2FQ9Z8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein tyrosine phosphatase type IVA 2 (Homo sapiens (Human)) | BDBM50059084 (CHEMBL3393171 | US10308663, Thienopyridone (5)) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 277 | n/a | n/a | n/a | n/a | n/a | n/a |
European Molecular Biology Laboratory Curated by ChEMBL | Assay Description Inhibition of human PRL2 expressed in Escherichia coli using TAMRA-Thr-Ala-Asp-Ile-Tyr(PO3H2)-Glu-NH2 substrate by immobilized metal ion affinity-bas... | Eur J Med Chem 88: 89-100 (2014) Article DOI: 10.1016/j.ejmech.2014.08.060 BindingDB Entry DOI: 10.7270/Q2FQ9Z8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine phosphatase 4A3 (Homo sapiens (Human)) | BDBM50059084 (CHEMBL3393171 | US10308663, Thienopyridone (5)) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
European Molecular Biology Laboratory Curated by ChEMBL | Assay Description Inhibition of full length human fusion His6 tagged PRL3 expressed in Escherichia coli using TAMRA-Thr-Ala-Asp-Ile-Tyr(PO3H2)-Glu-NH2 substrate by imm... | Eur J Med Chem 88: 89-100 (2014) Article DOI: 10.1016/j.ejmech.2014.08.060 BindingDB Entry DOI: 10.7270/Q2FQ9Z8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine phosphatase 4A3 (Homo sapiens (Human)) | BDBM50059084 (CHEMBL3393171 | US10308663, Thienopyridone (5)) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
European Molecular Biology Laboratory Curated by ChEMBL | Assay Description Inhibition of PRL3 (unknown origin) using DiFMUP as fluorogenic phosphate substrate assessed as DiFMUP dephosphorylation | Eur J Med Chem 88: 89-100 (2014) Article DOI: 10.1016/j.ejmech.2014.08.060 BindingDB Entry DOI: 10.7270/Q2FQ9Z8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine phosphatase 4A3 (Homo sapiens (Human)) | BDBM50059084 (CHEMBL3393171 | US10308663, Thienopyridone (5)) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh Curated by ChEMBL | Assay Description Inhibition of recombinant human His-6-tagged PTP4A3 expressed in Escherichia coli assessed as increase in polarization using TAMRAThr-Ala-Asp-Ile-Tyr... | Bioorg Med Chem Lett 29: 2008-2015 (2019) Article DOI: 10.1016/j.bmcl.2019.06.048 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein tyrosine phosphatase type IVA 1 (Homo sapiens (Human)) | BDBM50059084 (CHEMBL3393171 | US10308663, Thienopyridone (5)) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
European Molecular Biology Laboratory Curated by ChEMBL | Assay Description Inhibition of PRL1 (unknown origin) using DiFMUP as fluorogenic phosphate substrate assessed as DiFMUP dephosphorylation | Eur J Med Chem 88: 89-100 (2014) Article DOI: 10.1016/j.ejmech.2014.08.060 BindingDB Entry DOI: 10.7270/Q2FQ9Z8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine phosphatase 4A3 (Homo sapiens (Human)) | BDBM50059084 (CHEMBL3393171 | US10308663, Thienopyridone (5)) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The in vitro biochemical evaluation of all compounds was carried out using recombinant human PTP4A3, overexpressed as a His6-tag fusion protein in E.... | Bioorg Med Chem Lett 17: 4664-9 (2007) BindingDB Entry DOI: 10.7270/Q20867N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein tyrosine phosphatase type IVA 2 (Homo sapiens (Human)) | BDBM50059084 (CHEMBL3393171 | US10308663, Thienopyridone (5)) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh Curated by ChEMBL | Assay Description Inhibition of recombinant human His-6-tagged PTP4A2 expressed in Escherichia coli assessed as increase in polarization using TAMRAThr-Ala-Asp-Ile-Tyr... | Bioorg Med Chem Lett 29: 2008-2015 (2019) Article DOI: 10.1016/j.bmcl.2019.06.048 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein tyrosine phosphatase type IVA 1 (Homo sapiens (Human)) | BDBM50059084 (CHEMBL3393171 | US10308663, Thienopyridone (5)) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh Curated by ChEMBL | Assay Description Inhibition of recombinant human His-6-tagged PTP4A1 expressed in Escherichia coli assessed as increase in polarization using TAMRAThr-Ala-Asp-Ile-Tyr... | Bioorg Med Chem Lett 29: 2008-2015 (2019) Article DOI: 10.1016/j.bmcl.2019.06.048 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein tyrosine phosphatase type IVA 2 (Homo sapiens (Human)) | BDBM50059084 (CHEMBL3393171 | US10308663, Thienopyridone (5)) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.04E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
European Molecular Biology Laboratory Curated by ChEMBL | Assay Description Inhibition of PRL2 (unknown origin) using DiFMUP as fluorogenic phosphate substrate assessed as DiFMUP dephosphorylation | Eur J Med Chem 88: 89-100 (2014) Article DOI: 10.1016/j.ejmech.2014.08.060 BindingDB Entry DOI: 10.7270/Q2FQ9Z8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||