

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50074998 (E)-N-({[4-Methoxy-3-(2-methyl-quinolin-8-yloxymethyl)-2-propoxy-phenyl]-methyl-carbamoyl}-methyl)-3-(4-trifluoromethyl-phenyl)-acrylamide::CHEMBL324575
SMILES: CCCOc1c(COc2cccc3ccc(C)nc23)c(OC)ccc1N(C)C(=O)CNC(=O)\C=C\c1ccc(cc1)C(F)(F)F
InChI Key: InChIKey=QHVBUVLIEWZMCM-LDADJPATSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074998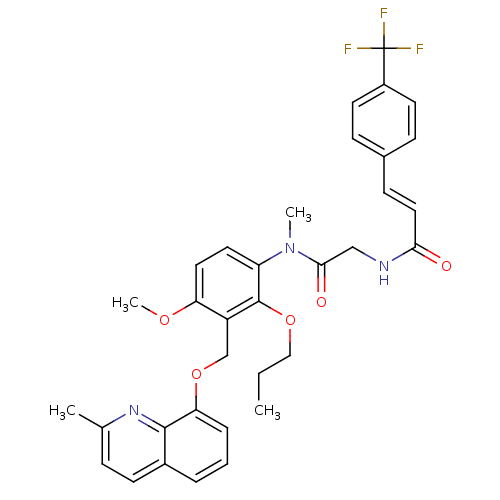 ((E)-N-({[4-Methoxy-3-(2-methyl-quinolin-8-yloxymet...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074998 ((E)-N-({[4-Methoxy-3-(2-methyl-quinolin-8-yloxymet...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of BK-induced (4x10E-8M) contraction of isolated guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074998 ((E)-N-({[4-Methoxy-3-(2-methyl-quinolin-8-yloxymet...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 220 | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of BK-induced (4x10E-8M) contraction of isolated guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||