

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50078688 3-(3,4-Difluoro-phenyl)-4-(4-methanesulfonyl-phenyl)-5H-furan-2-one::3-(3,4-difluorophenyl)-4-(4-(methylsulfonyl)phenyl)furan-2(5H)-one::CHEMBL18264
SMILES: CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccc(F)c(F)c1
InChI Key: InChIKey=INRQTVDUZFESAO-UHFFFAOYSA-N
Data: 12 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cyclooxygenase-1 (COX-1) (Homo sapiens (Human)) | BDBM50078688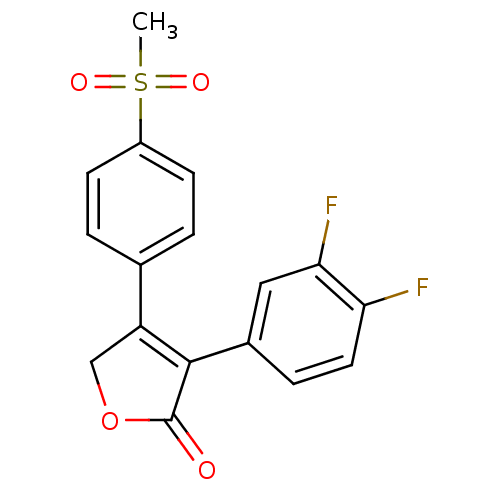 (3-(3,4-Difluoro-phenyl)-4-(4-methanesulfonyl-pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory potency against Prostaglandin G/H synthase 1 in human whole blood assay | Bioorg Med Chem Lett 14: 1201-3 (2004) Article DOI: 10.1016/j.bmcl.2003.12.047 BindingDB Entry DOI: 10.7270/Q27P8XTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase-1 (COX-1) (Homo sapiens (Human)) | BDBM50078688 (3-(3,4-Difluoro-phenyl)-4-(4-methanesulfonyl-pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against prostaglandin G/H synthase 1 in human whole blood | Bioorg Med Chem Lett 15: 3197-200 (2005) Article DOI: 10.1016/j.bmcl.2005.05.002 BindingDB Entry DOI: 10.7270/Q2ZW1KFH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase (Homo sapiens (Human)) | BDBM50078688 (3-(3,4-Difluoro-phenyl)-4-(4-methanesulfonyl-pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory potency against cyclooxygenase-2 in human whole blood assay | Bioorg Med Chem Lett 14: 1201-3 (2004) Article DOI: 10.1016/j.bmcl.2003.12.047 BindingDB Entry DOI: 10.7270/Q27P8XTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase (Homo sapiens (Human)) | BDBM50078688 (3-(3,4-Difluoro-phenyl)-4-(4-methanesulfonyl-pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against prostaglandin G/H synthase 2 in human whole blood | Bioorg Med Chem Lett 15: 3197-200 (2005) Article DOI: 10.1016/j.bmcl.2005.05.002 BindingDB Entry DOI: 10.7270/Q2ZW1KFH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase-1 (COX-1) (Homo sapiens (Human)) | BDBM50078688 (3-(3,4-Difluoro-phenyl)-4-(4-methanesulfonyl-pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against human whole cells Prostaglandin G/H synthase 1 | Bioorg Med Chem Lett 9: 1773-8 (1999) BindingDB Entry DOI: 10.7270/Q2QJ7HT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Cavia porcellus) | BDBM50078688 (3-(3,4-Difluoro-phenyl)-4-(4-methanesulfonyl-pheny...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard Curated by ChEMBL | Assay Description Inhibition of COX2 in guinea pig blood assessed as reduction of LPS-induced PGE2 accumulation at 10 to 10000 nM preincubated with LPS followed by com... | Eur J Med Chem 171: 66-92 (2019) Article DOI: 10.1016/j.ejmech.2019.03.021 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase/G/H synthase 2 (Homo sapiens (Human)) | BDBM50078688 (3-(3,4-Difluoro-phenyl)-4-(4-methanesulfonyl-pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against human whole blood Prostaglandin G/H synthase 1 | Bioorg Med Chem Lett 9: 1773-8 (1999) BindingDB Entry DOI: 10.7270/Q2QJ7HT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase (Homo sapiens (Human)) | BDBM50078688 (3-(3,4-Difluoro-phenyl)-4-(4-methanesulfonyl-pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against human whole blood Prostaglandin G/H synthase 1 | Bioorg Med Chem Lett 9: 1773-8 (1999) BindingDB Entry DOI: 10.7270/Q2QJ7HT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase/G/H synthase 2 (Homo sapiens (Human)) | BDBM50078688 (3-(3,4-Difluoro-phenyl)-4-(4-methanesulfonyl-pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory potency against Prostaglandin G/H synthase 1 in human whole blood assay | Bioorg Med Chem Lett 14: 1201-3 (2004) Article DOI: 10.1016/j.bmcl.2003.12.047 BindingDB Entry DOI: 10.7270/Q27P8XTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase (Homo sapiens (Human)) | BDBM50078688 (3-(3,4-Difluoro-phenyl)-4-(4-methanesulfonyl-pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human Prostaglandin G/H synthase 2 | Bioorg Med Chem Lett 14: 1201-3 (2004) Article DOI: 10.1016/j.bmcl.2003.12.047 BindingDB Entry DOI: 10.7270/Q27P8XTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Oryctolagus cuniculus) | BDBM50078688 (3-(3,4-Difluoro-phenyl)-4-(4-methanesulfonyl-pheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard Curated by ChEMBL | Assay Description Inhibition of COX2 in rabbit whole blood assessed as reduction of LPS-induced PGE2 accumulation at 10 to 10000 nM preincubated with LPS followed by c... | Eur J Med Chem 171: 66-92 (2019) Article DOI: 10.1016/j.ejmech.2019.03.021 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase (Homo sapiens (Human)) | BDBM50078688 (3-(3,4-Difluoro-phenyl)-4-(4-methanesulfonyl-pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against human whole cells (CHO) Prostaglandin G/H synthase 2 | Bioorg Med Chem Lett 9: 1773-8 (1999) BindingDB Entry DOI: 10.7270/Q2QJ7HT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||