

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50087007 8-(7-Chloro-1,2,3,4-tetrahydro-naphthalen-2-yl)-1-phenyl-1,3,8-triaza-spiro[4.5]decan-4-one::CHEMBL447779
SMILES: Clc1ccc2CCC(Cc2c1)N1CCC2(CC1)N(CNC2=O)c1ccccc1
InChI Key: InChIKey=XYFWBDPFFPDLGC-UHFFFAOYSA-N
Data: 4 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50087007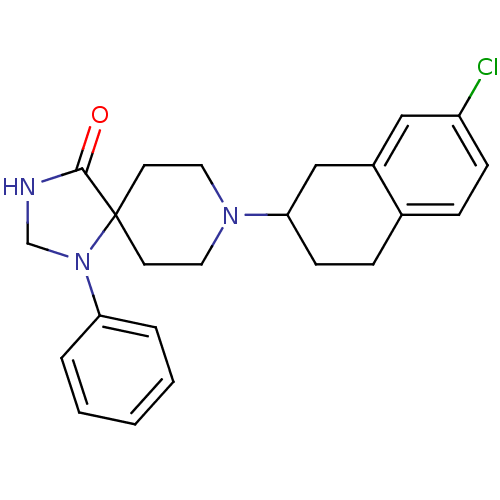 (8-(7-Chloro-1,2,3,4-tetrahydro-naphthalen-2-yl)-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Effective concentration required to stimulate binding of GTPgammaS to Opioid receptor like 1 was determined using scintillation proximity assay | J Med Chem 43: 1329-38 (2001) BindingDB Entry DOI: 10.7270/Q2ZG6SZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50087007 (8-(7-Chloro-1,2,3,4-tetrahydro-naphthalen-2-yl)-1-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Competitive binding displacement analyses was performed from permanently transfected HEK293 cells expressing human Opioid receptor mu 1 | J Med Chem 43: 1329-38 (2001) BindingDB Entry DOI: 10.7270/Q2ZG6SZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50087007 (8-(7-Chloro-1,2,3,4-tetrahydro-naphthalen-2-yl)-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Competitive binding displacement analyses was performed from permanently transfected HEK293 cells expressing Opioid receptor kappa 1 | J Med Chem 43: 1329-38 (2001) BindingDB Entry DOI: 10.7270/Q2ZG6SZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50087007 (8-(7-Chloro-1,2,3,4-tetrahydro-naphthalen-2-yl)-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Competitive binding displacement analyses was performed from permanently transfected HEK293 cells expressing human Opioid receptor delta 1 | J Med Chem 43: 1329-38 (2001) BindingDB Entry DOI: 10.7270/Q2ZG6SZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||