

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: NC(=N)c1ccc(Oc2ccc(Oc3ccc(cc3)C(N)=N)cc2)cc1
InChI Key: InChIKey=JARPXSXRDFAPLC-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50098553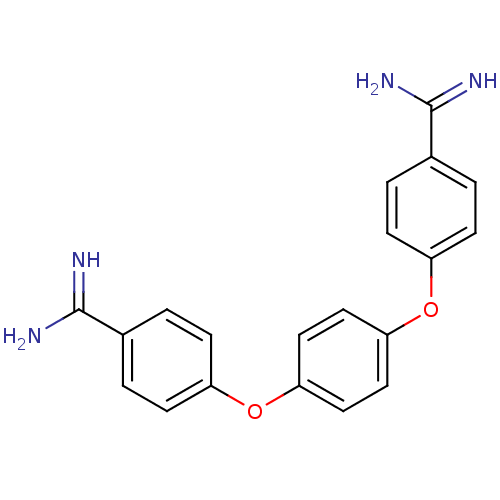 (4-(4-{4-[amino(imino)methyl]phenoxy}phenoxy)benzen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited Curated by ChEMBL | Assay Description Inhibition of recombinant matriptase (unknown origin) using Boc-Gln-Ala-Arg-7-amido-4-methyl coumarin hydrobromide | Bioorg Med Chem 22: 3187-203 (2014) Article DOI: 10.1016/j.bmc.2014.04.013 BindingDB Entry DOI: 10.7270/Q2J967ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50098553 (4-(4-{4-[amino(imino)methyl]phenoxy}phenoxy)benzen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 208 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description compound was tested for inhibitory activity against Matriptase | J Med Chem 44: 1349-55 (2001) BindingDB Entry DOI: 10.7270/Q2057F62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50098553 (4-(4-{4-[amino(imino)methyl]phenoxy}phenoxy)benzen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description compound was tested for inhibitory activity against Urokinase-type plasminogen activator(microPa) | J Med Chem 44: 1349-55 (2001) BindingDB Entry DOI: 10.7270/Q2057F62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50098553 (4-(4-{4-[amino(imino)methyl]phenoxy}phenoxy)benzen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description compound was tested for inhibitory activity against Thrombin | J Med Chem 44: 1349-55 (2001) BindingDB Entry DOI: 10.7270/Q2057F62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||