 Found 12 hits for monomerid = 50099692
Found 12 hits for monomerid = 50099692 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Leukocyte common antigen
(Homo sapiens (Human)) | BDBM50099692
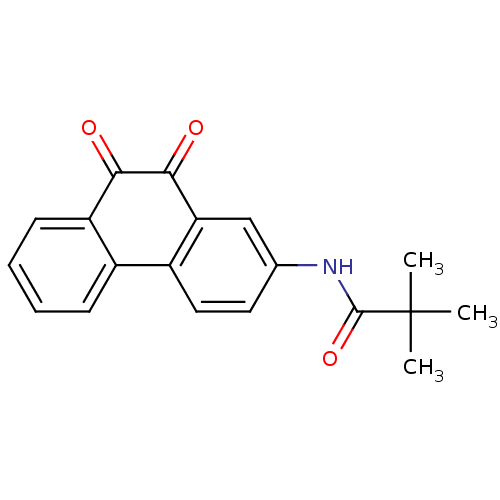
(CHEMBL51314 | N-(9,10-Dioxo-9,10-dihydro-phenanthr...)Show SMILES CC(C)(C)C(=O)Nc1ccc-2c(c1)C(=O)C(=O)c1ccccc-21 Show InChI InChI=1S/C19H17NO3/c1-19(2,3)18(23)20-11-8-9-13-12-6-4-5-7-14(12)16(21)17(22)15(13)10-11/h4-10H,1-3H3,(H,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
United States Army Medical Research Institute of Infectious Diseases
| Assay Description
Protein phosphatases were purchased from Upstate Biotechnology (Lake Placid, NY). |
J Biol Chem 284: 12874-85 (2009)
Article DOI: 10.1074/jbc.M809633200
BindingDB Entry DOI: 10.7270/Q2J67FJV |
More data for this
Ligand-Target Pair | |
Fas-associated protein-tyrosine phosphatase 1 (FAP1)
(Homo sapiens (Human)) | BDBM50099692

(CHEMBL51314 | N-(9,10-Dioxo-9,10-dihydro-phenanthr...)Show SMILES CC(C)(C)C(=O)Nc1ccc-2c(c1)C(=O)C(=O)c1ccccc-21 Show InChI InChI=1S/C19H17NO3/c1-19(2,3)18(23)20-11-8-9-13-12-6-4-5-7-14(12)16(21)17(22)15(13)10-11/h4-10H,1-3H3,(H,20,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against FAP-1 pNPP |
J Med Chem 44: 1777-93 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WQQ |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50099692

(CHEMBL51314 | N-(9,10-Dioxo-9,10-dihydro-phenanthr...)Show SMILES CC(C)(C)C(=O)Nc1ccc-2c(c1)C(=O)C(=O)c1ccccc-21 Show InChI InChI=1S/C19H17NO3/c1-19(2,3)18(23)20-11-8-9-13-12-6-4-5-7-14(12)16(21)17(22)15(13)10-11/h4-10H,1-3H3,(H,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Cathepsin S |
J Med Chem 44: 1777-93 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WQQ |
More data for this
Ligand-Target Pair | |
Leukocyte common antigen
(Homo sapiens (Human)) | BDBM50099692

(CHEMBL51314 | N-(9,10-Dioxo-9,10-dihydro-phenanthr...)Show SMILES CC(C)(C)C(=O)Nc1ccc-2c(c1)C(=O)C(=O)c1ccccc-21 Show InChI InChI=1S/C19H17NO3/c1-19(2,3)18(23)20-11-8-9-13-12-6-4-5-7-14(12)16(21)17(22)15(13)10-11/h4-10H,1-3H3,(H,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against CD45 protein-tyrosine phosphatase using lck-10 mer as substrate |
J Med Chem 44: 1777-93 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WQQ |
More data for this
Ligand-Target Pair | |
Fas-associated protein-tyrosine phosphatase 1 (FAP1)
(Homo sapiens (Human)) | BDBM50099692

(CHEMBL51314 | N-(9,10-Dioxo-9,10-dihydro-phenanthr...)Show SMILES CC(C)(C)C(=O)Nc1ccc-2c(c1)C(=O)C(=O)c1ccccc-21 Show InChI InChI=1S/C19H17NO3/c1-19(2,3)18(23)20-11-8-9-13-12-6-4-5-7-14(12)16(21)17(22)15(13)10-11/h4-10H,1-3H3,(H,20,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against FAP-1 using lck-10 mer as substrate |
J Med Chem 44: 1777-93 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WQQ |
More data for this
Ligand-Target Pair | |
Fas-associated protein-tyrosine phosphatase 1 (FAP1)
(Homo sapiens (Human)) | BDBM50099692

(CHEMBL51314 | N-(9,10-Dioxo-9,10-dihydro-phenanthr...)Show SMILES CC(C)(C)C(=O)Nc1ccc-2c(c1)C(=O)C(=O)c1ccccc-21 Show InChI InChI=1S/C19H17NO3/c1-19(2,3)18(23)20-11-8-9-13-12-6-4-5-7-14(12)16(21)17(22)15(13)10-11/h4-10H,1-3H3,(H,20,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of protein tyrosine phosphatase N13 (unknown origin) phosphorylation |
J Biol Chem 282: 35361-72 (2007)
Article DOI: 10.1074/jbc.M706923200
BindingDB Entry DOI: 10.7270/Q25M65HF |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50099692

(CHEMBL51314 | N-(9,10-Dioxo-9,10-dihydro-phenanthr...)Show SMILES CC(C)(C)C(=O)Nc1ccc-2c(c1)C(=O)C(=O)c1ccccc-21 Show InChI InChI=1S/C19H17NO3/c1-19(2,3)18(23)20-11-8-9-13-12-6-4-5-7-14(12)16(21)17(22)15(13)10-11/h4-10H,1-3H3,(H,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Cathepsin B |
J Med Chem 44: 1777-93 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WQQ |
More data for this
Ligand-Target Pair | |
Leukocyte common antigen
(Homo sapiens (Human)) | BDBM50099692

(CHEMBL51314 | N-(9,10-Dioxo-9,10-dihydro-phenanthr...)Show SMILES CC(C)(C)C(=O)Nc1ccc-2c(c1)C(=O)C(=O)c1ccccc-21 Show InChI InChI=1S/C19H17NO3/c1-19(2,3)18(23)20-11-8-9-13-12-6-4-5-7-14(12)16(21)17(22)15(13)10-11/h4-10H,1-3H3,(H,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against the cytosolic portion of CD45 protein-tyrosine phosphatase using pNPP as the substrate |
J Med Chem 44: 1777-93 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WQQ |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50099692

(CHEMBL51314 | N-(9,10-Dioxo-9,10-dihydro-phenanthr...)Show SMILES CC(C)(C)C(=O)Nc1ccc-2c(c1)C(=O)C(=O)c1ccccc-21 Show InChI InChI=1S/C19H17NO3/c1-19(2,3)18(23)20-11-8-9-13-12-6-4-5-7-14(12)16(21)17(22)15(13)10-11/h4-10H,1-3H3,(H,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Cathepsin L |
J Med Chem 44: 1777-93 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WQQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50099692

(CHEMBL51314 | N-(9,10-Dioxo-9,10-dihydro-phenanthr...)Show SMILES CC(C)(C)C(=O)Nc1ccc-2c(c1)C(=O)C(=O)c1ccccc-21 Show InChI InChI=1S/C19H17NO3/c1-19(2,3)18(23)20-11-8-9-13-12-6-4-5-7-14(12)16(21)17(22)15(13)10-11/h4-10H,1-3H3,(H,20,23) | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of Lck (unknown origin) phosphorylation |
J Biol Chem 282: 35361-72 (2007)
Article DOI: 10.1074/jbc.M706923200
BindingDB Entry DOI: 10.7270/Q25M65HF |
More data for this
Ligand-Target Pair | |
Protein-tyrosine phosphatase 1B
(Homo sapiens (Human)) | BDBM50099692

(CHEMBL51314 | N-(9,10-Dioxo-9,10-dihydro-phenanthr...)Show SMILES CC(C)(C)C(=O)Nc1ccc-2c(c1)C(=O)C(=O)c1ccccc-21 Show InChI InChI=1S/C19H17NO3/c1-19(2,3)18(23)20-11-8-9-13-12-6-4-5-7-14(12)16(21)17(22)15(13)10-11/h4-10H,1-3H3,(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of protein tyrosine phosphatase N1 (unknown origin) phosphorylation |
J Biol Chem 282: 35361-72 (2007)
Article DOI: 10.1074/jbc.M706923200
BindingDB Entry DOI: 10.7270/Q25M65HF |
More data for this
Ligand-Target Pair | |
Protein-tyrosine phosphatase 1B
(Homo sapiens (Human)) | BDBM50099692

(CHEMBL51314 | N-(9,10-Dioxo-9,10-dihydro-phenanthr...)Show SMILES CC(C)(C)C(=O)Nc1ccc-2c(c1)C(=O)C(=O)c1ccccc-21 Show InChI InChI=1S/C19H17NO3/c1-19(2,3)18(23)20-11-8-9-13-12-6-4-5-7-14(12)16(21)17(22)15(13)10-11/h4-10H,1-3H3,(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against PTP1B using lck as substrate |
J Med Chem 44: 1777-93 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WQQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data