

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50105890 12-Amino-6-(3-guanidino-propyl)-3-(1H-indol-3-ylmethyl)-9-naphthalen-2-ylmethyl-2,5,8,11,14-pentaoxo-1,4,7,10,15pentaaza-cycloicosane-20-carboxylic acid::CHEMBL433645
SMILES: N[C@H]1CC(=O)NCCCC[C@@H](NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccc3ccccc3c2)NC1=O)C(N)=O
InChI Key: InChIKey=WMMWJBFBADMJIK-ZQKWFRRKSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50105890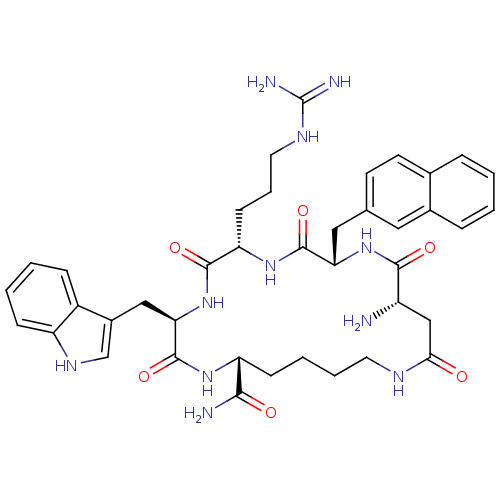 (12-Amino-6-(3-guanidino-propyl)-3-(1H-indol-3-ylme...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against human melanocortin receptor 4 (hMC4R) (concentration of the peptide at 50% specific binding) | J Med Chem 44: 3665-72 (2001) BindingDB Entry DOI: 10.7270/Q2RF5T9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (MC5R) (Homo sapiens (Human)) | BDBM50105890 (12-Amino-6-(3-guanidino-propyl)-3-(1H-indol-3-ylme...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against human melanocortin receptor 5 (hMC5R) (concentration of the peptide at 50% specific binding) | J Med Chem 44: 3665-72 (2001) BindingDB Entry DOI: 10.7270/Q2RF5T9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50105890 (12-Amino-6-(3-guanidino-propyl)-3-(1H-indol-3-ylme...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against human melanocortin receptor 3 (hMC3R) (concentration of the peptide at 50% specific binding) | J Med Chem 44: 3665-72 (2001) BindingDB Entry DOI: 10.7270/Q2RF5T9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (MC5R) (Homo sapiens (Human)) | BDBM50105890 (12-Amino-6-(3-guanidino-propyl)-3-(1H-indol-3-ylme...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Increase in intracellular cAMP in CHO cells expressing human melanocortin receptor 5. | J Med Chem 44: 3665-72 (2001) BindingDB Entry DOI: 10.7270/Q2RF5T9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||