

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50112358 CHEMBL3609374
SMILES: OS(=O)(=O)C(C(=O)Nc1ccc(NC(=O)C(=O)Nc2ccc(cc2)-c2ccccc2)cc1)c1ccccc1
InChI Key: InChIKey=UNNZCNDZZKUQJC-UHFFFAOYSA-N
Data: 16 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein phosphatase 5 (PP5) (Homo sapiens (Human)) | BDBM50112358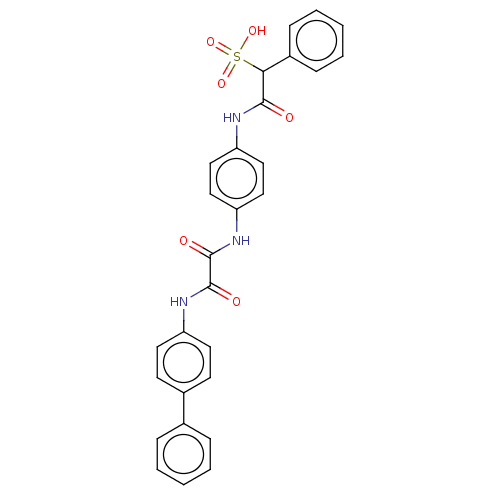 (CHEMBL3609374) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of phosphatase activity of human PP5 using pNPP as a substrate after 10 mins by spectrophotometer analysis | ACS Med Chem Lett 6: 782-6 (2015) BindingDB Entry DOI: 10.7270/Q2251M0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50112358 (CHEMBL3609374) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of phosphatase activity of SHP2 (unknown origin) using pNPP as a substrate after 10 mins by spectrophotometer analysis | ACS Med Chem Lett 6: 782-6 (2015) BindingDB Entry DOI: 10.7270/Q2251M0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase CDC14A (Homo sapiens (Human)) | BDBM50112358 (CHEMBL3609374) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of phosphatase activity of human CDC14A using pNPP as a substrate after 10 mins by spectrophotometer analysis | ACS Med Chem Lett 6: 782-6 (2015) BindingDB Entry DOI: 10.7270/Q2251M0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase (VHR) (Homo sapiens (Human)) | BDBM50112358 (CHEMBL3609374) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of phosphatase activity of human VHR using pNPP as a substrate after 10 mins by spectrophotometer analysis | ACS Med Chem Lett 6: 782-6 (2015) BindingDB Entry DOI: 10.7270/Q2251M0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase F (LAR) (Homo sapiens (Human)) | BDBM50112358 (CHEMBL3609374) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of phosphatase activity of human LAR using pNPP as a substrate after 10 mins by spectrophotometer analysis | ACS Med Chem Lett 6: 782-6 (2015) BindingDB Entry DOI: 10.7270/Q2251M0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase mu (PTPμ) (Homo sapiens (Human)) | BDBM50112358 (CHEMBL3609374) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of phosphatase activity of human PTPmu using pNPP as a substrate after 10 mins by spectrophotometer analysis | ACS Med Chem Lett 6: 782-6 (2015) BindingDB Entry DOI: 10.7270/Q2251M0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase gamma (Homo sapiens (Human)) | BDBM50112358 (CHEMBL3609374) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of phosphatase activity of human PTPgamma using pNPP as a substrate after 10 mins by spectrophotometer analysis | ACS Med Chem Lett 6: 782-6 (2015) BindingDB Entry DOI: 10.7270/Q2251M0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase epsilon (PTPε) (Homo sapiens (Human)) | BDBM50112358 (CHEMBL3609374) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of phosphatase activity of human PTPepsilon using pNPP as a substrate after 10 mins by spectrophotometer analysis | ACS Med Chem Lett 6: 782-6 (2015) BindingDB Entry DOI: 10.7270/Q2251M0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (PTPβ) (Homo sapiens (Human)) | BDBM50112358 (CHEMBL3609374) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of phosphatase activity of human PTPbeta using pNPP as a substrate after 10 mins by spectrophotometer analysis | ACS Med Chem Lett 6: 782-6 (2015) BindingDB Entry DOI: 10.7270/Q2251M0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine phosphatase alpha (Homo sapiens (Human)) | BDBM50112358 (CHEMBL3609374) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of phosphatase activity of human PTPalpha using pNPP as a substrate after 10 mins by spectrophotometer analysis | ACS Med Chem Lett 6: 782-6 (2015) BindingDB Entry DOI: 10.7270/Q2251M0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine phosphatase MEG2 (PTP-Meg2) (Homo sapiens (Human)) | BDBM50112358 (CHEMBL3609374) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of phosphatase activity of human Meg2 using pNPP as a substrate after 10 mins by spectrophotometer analysis | ACS Med Chem Lett 6: 782-6 (2015) BindingDB Entry DOI: 10.7270/Q2251M0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic protein-tyrosine phosphatase (HEPTP) (Homo sapiens (Human)) | BDBM50112358 (CHEMBL3609374) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of phosphatase activity of human HePTP using pNPP as a substrate after 10 mins by spectrophotometer analysis | ACS Med Chem Lett 6: 782-6 (2015) BindingDB Entry DOI: 10.7270/Q2251M0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic cell protein-tyrosine phosphatase 70Z-PEP (Homo sapiens (Human)) | BDBM50112358 (CHEMBL3609374) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of phosphatase activity of human LYP using pNPP as a substrate after 10 mins by spectrophotometer analysis | ACS Med Chem Lett 6: 782-6 (2015) BindingDB Entry DOI: 10.7270/Q2251M0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine phosphatase 1B (Homo sapiens (Human)) | BDBM50112358 (CHEMBL3609374) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of phosphatase activity of human PTP1B using pNPP as a substrate after 10 mins by spectrophotometer analysis | ACS Med Chem Lett 6: 782-6 (2015) BindingDB Entry DOI: 10.7270/Q2251M0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine phosphatase 1C (Homo sapiens (Human)) | BDBM50112358 (CHEMBL3609374) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of phosphatase activity of human SHP1 using pNPP as a substrate after 10 mins by spectrophotometer analysis | ACS Med Chem Lett 6: 782-6 (2015) BindingDB Entry DOI: 10.7270/Q2251M0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Low molecular weight phosphotyrosine protein phosphatase (Homo sapiens (Human)) | BDBM50112358 (CHEMBL3609374) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of phosphatase activity of human LMWPTP using pNPP as a substrate after 10 mins by spectrophotometer analysis | ACS Med Chem Lett 6: 782-6 (2015) BindingDB Entry DOI: 10.7270/Q2251M0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||