

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50113835 4-Fluoro-benzylamine::4-fluorobenzylamine::CHEMBL12392::Integrase inhibitor, R1{2}
SMILES: NCc1ccc(F)cc1
InChI Key: InChIKey=IIFVWLUQBAIPMJ-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine protease 1 (Homo sapiens (Human)) | BDBM50113835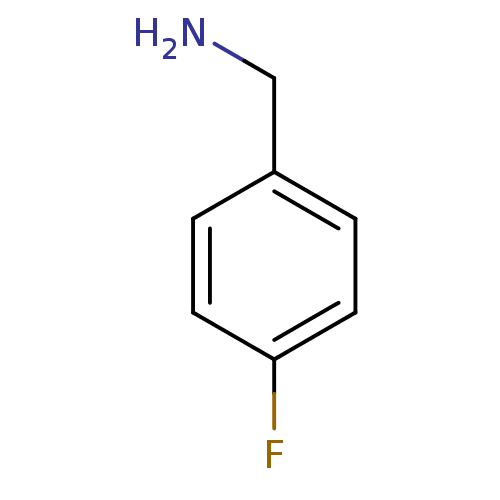 (4-Fluoro-benzylamine | 4-fluorobenzylamine | CHEMB...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity against bovine trypsin | J Med Chem 45: 2469-83 (2002) BindingDB Entry DOI: 10.7270/Q28916J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50113835 (4-Fluoro-benzylamine | 4-fluorobenzylamine | CHEMB...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | 4.27E+5 | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Binding affinity against trypsin | J Med Chem 45: 2770-80 (2002) Article DOI: 10.1021/jm0105833 BindingDB Entry DOI: 10.7270/Q2MG7S8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT8 (Homo sapiens (Human)) | BDBM50113835 (4-Fluoro-benzylamine | 4-fluorobenzylamine | CHEMB...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged KAT8 catalytic domain (125 to 458 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using SGRGK... | Eur J Med Chem 136: 480-486 (2017) Article DOI: 10.1016/j.ejmech.2017.05.015 BindingDB Entry DOI: 10.7270/Q20Z75S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50113835 (4-Fluoro-benzylamine | 4-fluorobenzylamine | CHEMB...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 4.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb | Assay Description Inhibition assay using HIV-1 integrase. | J Comb Chem 12: 84-90 (2010) Article DOI: 10.1021/cc9001026 BindingDB Entry DOI: 10.7270/Q2Q81BNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysyl oxidase homolog 2 (Homo sapiens (Human)) | BDBM50113835 (4-Fluoro-benzylamine | 4-fluorobenzylamine | CHEMB...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmAkea Inc. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human LOXL2 expressed in CHO cells assessed as reduction in H2O2 production using DAP as substrate preincubated... | ACS Med Chem Lett 8: 423-427 (2017) BindingDB Entry DOI: 10.7270/Q2542QV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||