

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: Nc1nc(c(s1)-c1ccnc2ccccc12)-c1ccccc1F
InChI Key: InChIKey=XFRSTLMKCGEHNT-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50151356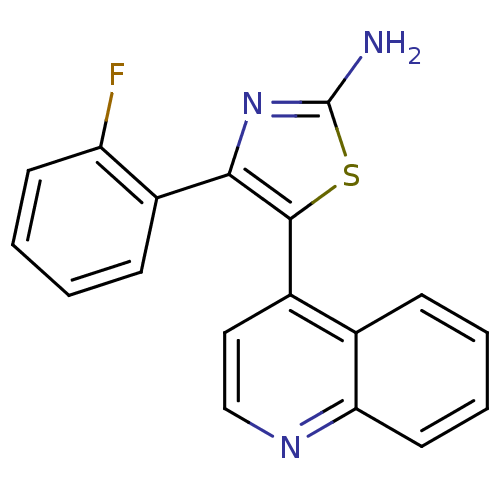 (4-(2-Fluoro-phenyl)-5-quinolin-4-yl-thiazol-2-ylam...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Stimulation of Transforming growth factor beta receptor I kinase in HepG2 cells | J Med Chem 47: 4494-506 (2004) Article DOI: 10.1021/jm0400247 BindingDB Entry DOI: 10.7270/Q2VQ325T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50151356 (4-(2-Fluoro-phenyl)-5-quinolin-4-yl-thiazol-2-ylam...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of TGFR1 | Eur J Med Chem 44: 4259-65 (2009) Article DOI: 10.1016/j.ejmech.2009.07.008 BindingDB Entry DOI: 10.7270/Q2BC3ZM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50151356 (4-(2-Fluoro-phenyl)-5-quinolin-4-yl-thiazol-2-ylam...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity to Activin A receptor type II-like kinase (ALK5) in fluorescence polarization binding assay; Range is (0.356-4.455) | J Med Chem 47: 4494-506 (2004) Article DOI: 10.1021/jm0400247 BindingDB Entry DOI: 10.7270/Q2VQ325T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||