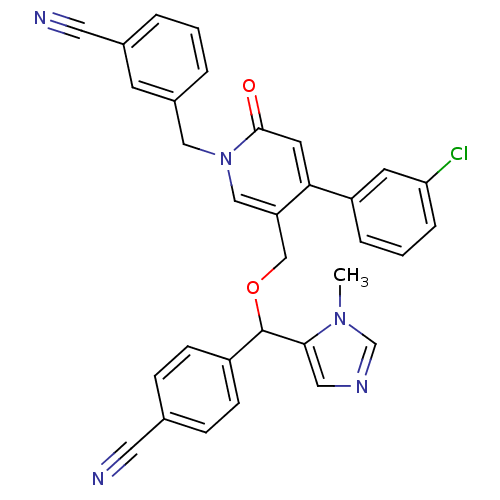
BDBM50151520 3-{4-(3-chlorophenyl)-5-[4-cyanophenyl(1-methyl-1H-5-imidazolyl)methoxymethyl]-2-oxo-1,2-dihydro-1-pyridinylmethyl}benzonitrile::CHEMBL365269
SMILES: Cn1cncc1C(OCc1cn(Cc2cccc(c2)C#N)c(=O)cc1-c1cccc(Cl)c1)c1ccc(cc1)C#N
InChI Key: InChIKey=XLADCPZMAAXJBZ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50151520
Found 2 hits for monomerid = 50151520 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Mus musculus) | BDBM50151520
(3-{4-(3-chlorophenyl)-5-[4-cyanophenyl(1-methyl-1H...)Show SMILES Cn1cncc1C(OCc1cn(Cc2cccc(c2)C#N)c(=O)cc1-c1cccc(Cl)c1)c1ccc(cc1)C#N Show InChI InChI=1S/C32H24ClN5O2/c1-37-21-36-17-30(37)32(25-10-8-22(15-34)9-11-25)40-20-27-19-38(18-24-5-2-4-23(12-24)16-35)31(39)14-29(27)26-6-3-7-28(33)13-26/h2-14,17,19,21,32H,18,20H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Factor Xa |
Bioorg Med Chem Lett 14: 4603-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.004
BindingDB Entry DOI: 10.7270/Q27080WG |
More data for this
Ligand-Target Pair | |
Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50151520

(3-{4-(3-chlorophenyl)-5-[4-cyanophenyl(1-methyl-1H...)Show SMILES Cn1cncc1C(OCc1cn(Cc2cccc(c2)C#N)c(=O)cc1-c1cccc(Cl)c1)c1ccc(cc1)C#N Show InChI InChI=1S/C32H24ClN5O2/c1-37-21-36-17-30(37)32(25-10-8-22(15-34)9-11-25)40-20-27-19-38(18-24-5-2-4-23(12-24)16-35)31(39)14-29(27)26-6-3-7-28(33)13-26/h2-14,17,19,21,32H,18,20H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Transcriptional repression in HepG2 cells expressing human glucocorticoid receptor |
Bioorg Med Chem Lett 14: 4603-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.004
BindingDB Entry DOI: 10.7270/Q27080WG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data