

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50158735 3-(3-{5-[1-(4-Ethoxy-phenyl)-meth-(Z)-ylidene]-4-oxo-2-thioxo-thiazolidin-3-yl}-propionylamino)-benzoic acid::CHEMBL414840::cid_1580464
SMILES: CCOc1ccc(\C=C2/SC(=S)N(CCC(=O)Nc3cccc(c3)C(O)=O)C2=O)cc1
InChI Key: InChIKey=KVETYKHCSWJCBQ-PDGQHHTCSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| carboxy-terminal domain RNA polymerase II polypeptide A small phosphatase 1 isoform 1 (Homo sapiens (Human)) | BDBM50158735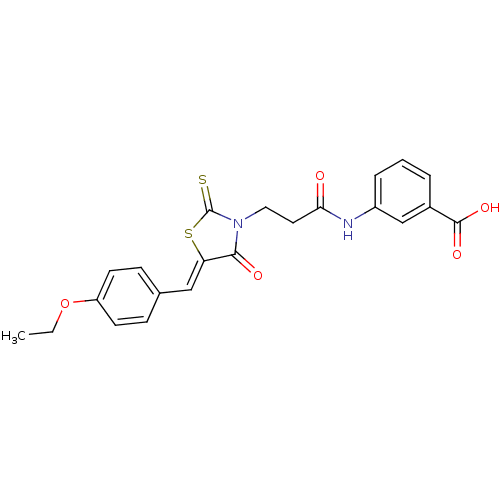 (3-(3-{5-[1-(4-Ethoxy-phenyl)-meth-(Z)-ylidene]-4-o...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 3.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2V986KM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 reverse transcriptase (Human immunodeficiency virus 1) | BDBM50158735 (3-(3-{5-[1-(4-Ethoxy-phenyl)-meth-(Z)-ylidene]-4-o...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California Curated by ChEMBL | Assay Description Inhibitory concentration against human immuno deficiency virus type 1 integrase (3'-processing) | J Med Chem 48: 111-20 (2005) Article DOI: 10.1021/jm0496077 BindingDB Entry DOI: 10.7270/Q28S4PD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 reverse transcriptase (Human immunodeficiency virus 1) | BDBM50158735 (3-(3-{5-[1-(4-Ethoxy-phenyl)-meth-(Z)-ylidene]-4-o...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California Curated by ChEMBL | Assay Description Inhibitory concentration against human immuno deficiency virus type 1 integrase (Strand Transfer) | J Med Chem 48: 111-20 (2005) Article DOI: 10.1021/jm0496077 BindingDB Entry DOI: 10.7270/Q28S4PD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||