

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50159154 CHEMBL3787379
SMILES: OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN\C(N)=N\C(=O)NCCOCCOCCNC(=O)CC)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O
InChI Key: InChIKey=JOKUSRASTVQLGX-NLWCPLLQSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin II receptor (Homo sapiens (Human)) | BDBM50159154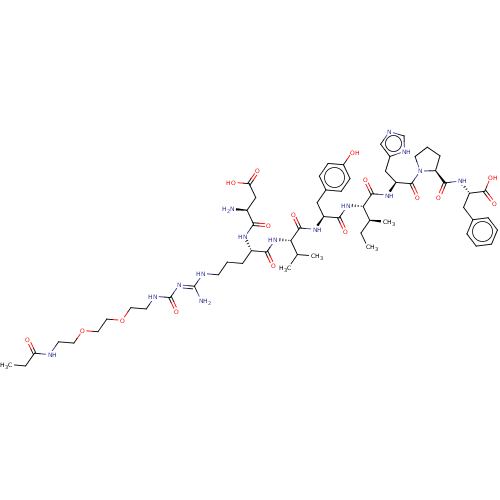 (CHEMBL3787379) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-Asp-{Nomega-[N-(4-propanoylaminobutyl)aminocarbonyl]}Arg-ValTyr-Ile-His-Pro-Phe-OH Tris(hydrotrifluoroacetate) from human AT1 re... | J Med Chem 59: 1925-45 (2016) BindingDB Entry DOI: 10.7270/Q2V69MGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin II receptor (Homo sapiens (Human)) | BDBM50159154 (CHEMBL3787379) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Agonist activity at human AT1 receptor transfected in CHO cells co-expressing Galpha16-mtAEQ assessed as induction of intracellular Ca2+ mobilization... | J Med Chem 59: 1925-45 (2016) BindingDB Entry DOI: 10.7270/Q2V69MGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin II receptor (Homo sapiens (Human)) | BDBM50159154 (CHEMBL3787379) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Agonist activity at human AT1 receptor transfected in CHO cells co-expressing Galpha16-mtAEQ assessed as induction of intracellular Ca2+ mobilization... | J Med Chem 59: 1925-45 (2016) BindingDB Entry DOI: 10.7270/Q2V69MGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||