

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50202656 CHEMBL3916438::US10730889, Example 419
SMILES: CC1(COC1)OC(=O)N1CCC2(C[C@@H]2CNC(=O)N2Cc3ccncc3C2)CC1
InChI Key: InChIKey=QFORHKAMQGBXQZ-QGZVFWFLSA-N
Data: 11 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50202656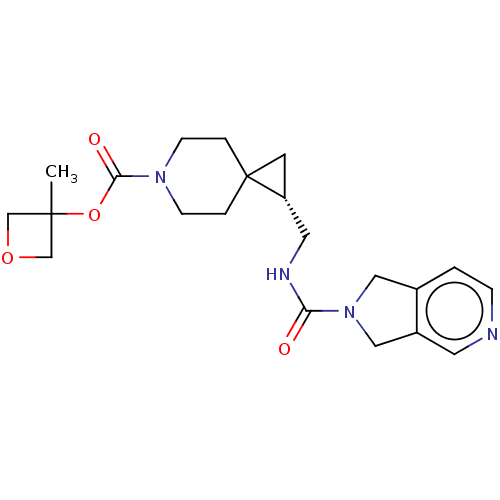 (CHEMBL3916438 | US10730889, Example 419) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Reversible inhibition of CYP3A4 in human liver microsomes using testosterone as substrate in presence of NADPH by LC-MS/MS analysis | J Med Chem 59: 8345-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00697 BindingDB Entry DOI: 10.7270/Q2KW5J0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50202656 (CHEMBL3916438 | US10730889, Example 419) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of human full length C-terminal His6-tagged NAMPT expressed in Escherichia coli Rosetta (DE3) cells using nicotinamide as substrate incuba... | J Med Chem 59: 8345-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00697 BindingDB Entry DOI: 10.7270/Q2KW5J0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50202656 (CHEMBL3916438 | US10730889, Example 419) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of NAMPT in human HT1080 cells assessed as decrease in cell viability after 96 hrs by CyQuant-Direct reagent based assay | J Med Chem 59: 8345-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00697 BindingDB Entry DOI: 10.7270/Q2KW5J0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50202656 (CHEMBL3916438 | US10730889, Example 419) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Reversible inhibition of CYP2D6 in human liver microsomes using (S)-warfarin as substrate in presence of NADPH by LC-MS/MS analysis | J Med Chem 59: 8345-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00697 BindingDB Entry DOI: 10.7270/Q2KW5J0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A (Homo sapiens (Human)) | BDBM50202656 (CHEMBL3916438 | US10730889, Example 419) | PDB MMDB Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Reversible inhibition of CYP1A2 in human liver microsomes using (S)-warfarin as substrate in presence of NADPH by LC-MS/MS analysis | J Med Chem 59: 8345-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00697 BindingDB Entry DOI: 10.7270/Q2KW5J0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50202656 (CHEMBL3916438 | US10730889, Example 419) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.49 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA TM, LLC; Genentech, Inc. US Patent | Assay Description NAMPT Protein Purification. Recombinant His-tagged NAMPT was produced in E. coli cells, purified over a Ni column, and further purified over a size-e... | US Patent US10730889 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50202656 (CHEMBL3916438 | US10730889, Example 419) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of NAMPT in human HT1080 cells assessed as decrease in cell viability by measuring plasma protein binding corrected IC50 after 96 hrs by C... | J Med Chem 59: 8345-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00697 BindingDB Entry DOI: 10.7270/Q2KW5J0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50202656 (CHEMBL3916438 | US10730889, Example 419) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of NAMPT in human A2780 cells assessed as decrease in cell viability after 72 hrs by SRB assay | J Med Chem 59: 8345-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00697 BindingDB Entry DOI: 10.7270/Q2KW5J0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50202656 (CHEMBL3916438 | US10730889, Example 419) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Reversible inhibition of CYP3A4 in human liver microsomes using midazolam as substrate in presence of NADPH by LC-MS/MS analysis | J Med Chem 59: 8345-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00697 BindingDB Entry DOI: 10.7270/Q2KW5J0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50202656 (CHEMBL3916438 | US10730889, Example 419) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Reversible inhibition of CYP2C9 in human liver microsomes using (S)-warfarin as substrate in presence of NADPH by LC-MS/MS analysis | J Med Chem 59: 8345-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00697 BindingDB Entry DOI: 10.7270/Q2KW5J0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50202656 (CHEMBL3916438 | US10730889, Example 419) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Reversible inhibition of CYP2C19 in human liver microsomes using (S)-warfarin as substrate in presence of NADPH by LC-MS/MS analysis | J Med Chem 59: 8345-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00697 BindingDB Entry DOI: 10.7270/Q2KW5J0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||