 Found 12 hits for monomerid = 50232478
Found 12 hits for monomerid = 50232478 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50232478
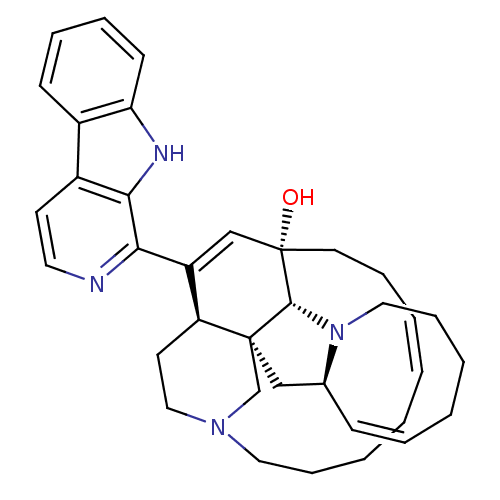
(CHEMBL403233)Show SMILES O[C@@]12CC\C=C/CCCCN3CC[C@@H](C(=C1)c1nccc4c5ccccc5[nH]c14)[C@]1(C[C@@H]4\C=C/CCCCN4[C@@H]21)C3 |r,c:4,14,36| Show InChI InChI=1S/C36H44N4O/c41-36-18-10-4-1-2-5-11-20-39-22-17-30(35(25-39)23-26-13-7-3-6-12-21-40(26)34(35)36)29(24-36)32-33-28(16-19-37-32)27-14-8-9-15-31(27)38-33/h1,4,7-9,13-16,19,24,26,30,34,38,41H,2-3,5-6,10-12,17-18,20-23,25H2/b4-1-,13-7-/t26-,30-,34+,35-,36-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Louisiana at Monroe
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GSK3beta Z'-LYTE kinase assay kit method |
J Med Chem 53: 8534-45 (2010)
Article DOI: 10.1021/jm100941j
BindingDB Entry DOI: 10.7270/Q2DB8230 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50232478

(CHEMBL403233)Show SMILES O[C@@]12CC\C=C/CCCCN3CC[C@@H](C(=C1)c1nccc4c5ccccc5[nH]c14)[C@]1(C[C@@H]4\C=C/CCCCN4[C@@H]21)C3 |r,c:4,14,36| Show InChI InChI=1S/C36H44N4O/c41-36-18-10-4-1-2-5-11-20-39-22-17-30(35(25-39)23-26-13-7-3-6-12-21-40(26)34(35)36)29(24-36)32-33-28(16-19-37-32)27-14-8-9-15-31(27)38-33/h1,4,7-9,13-16,19,24,26,30,34,38,41H,2-3,5-6,10-12,17-18,20-23,25H2/b4-1-,13-7-/t26-,30-,34+,35-,36-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSKbeta |
J Nat Prod 70: 1397-405 (2007)
Article DOI: 10.1021/np060092r
BindingDB Entry DOI: 10.7270/Q2R78G2K |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50232478

(CHEMBL403233)Show SMILES O[C@@]12CC\C=C/CCCCN3CC[C@@H](C(=C1)c1nccc4c5ccccc5[nH]c14)[C@]1(C[C@@H]4\C=C/CCCCN4[C@@H]21)C3 |r,c:4,14,36| Show InChI InChI=1S/C36H44N4O/c41-36-18-10-4-1-2-5-11-20-39-22-17-30(35(25-39)23-26-13-7-3-6-12-21-40(26)34(35)36)29(24-36)32-33-28(16-19-37-32)27-14-8-9-15-31(27)38-33/h1,4,7-9,13-16,19,24,26,30,34,38,41H,2-3,5-6,10-12,17-18,20-23,25H2/b4-1-,13-7-/t26-,30-,34+,35-,36-/m0/s1 | PDB
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of human microsomal ACAT2 overexpressed in CHO cells using [14C]oleoyl-CoA as substrate assessed as formation of cholester... |
Bioorg Med Chem 21: 3831-8 (2013)
Article DOI: 10.1016/j.bmc.2013.04.025
BindingDB Entry DOI: 10.7270/Q2PV6MR1 |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50232478

(CHEMBL403233)Show SMILES O[C@@]12CC\C=C/CCCCN3CC[C@@H](C(=C1)c1nccc4c5ccccc5[nH]c14)[C@]1(C[C@@H]4\C=C/CCCCN4[C@@H]21)C3 |r,c:4,14,36| Show InChI InChI=1S/C36H44N4O/c41-36-18-10-4-1-2-5-11-20-39-22-17-30(35(25-39)23-26-13-7-3-6-12-21-40(26)34(35)36)29(24-36)32-33-28(16-19-37-32)27-14-8-9-15-31(27)38-33/h1,4,7-9,13-16,19,24,26,30,34,38,41H,2-3,5-6,10-12,17-18,20-23,25H2/b4-1-,13-7-/t26-,30-,34+,35-,36-/m0/s1 | PDB
KEGG
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of human microsomal ACAT1 overexpressed in CHO cells using [14C]oleoyl-CoA as substrate assessed as formation of cholester... |
Bioorg Med Chem 21: 3831-8 (2013)
Article DOI: 10.1016/j.bmc.2013.04.025
BindingDB Entry DOI: 10.7270/Q2PV6MR1 |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50232478

(CHEMBL403233)Show SMILES O[C@@]12CC\C=C/CCCCN3CC[C@@H](C(=C1)c1nccc4c5ccccc5[nH]c14)[C@]1(C[C@@H]4\C=C/CCCCN4[C@@H]21)C3 |r,c:4,14,36| Show InChI InChI=1S/C36H44N4O/c41-36-18-10-4-1-2-5-11-20-39-22-17-30(35(25-39)23-26-13-7-3-6-12-21-40(26)34(35)36)29(24-36)32-33-28(16-19-37-32)27-14-8-9-15-31(27)38-33/h1,4,7-9,13-16,19,24,26,30,34,38,41H,2-3,5-6,10-12,17-18,20-23,25H2/b4-1-,13-7-/t26-,30-,34+,35-,36-/m0/s1 | PDB
KEGG
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of microsomal ACAT in human MDM using [14C]oleoyl-CoA as substrate assessed as formation of cholesteryl [14C]-oleate after... |
Bioorg Med Chem 21: 3831-8 (2013)
Article DOI: 10.1016/j.bmc.2013.04.025
BindingDB Entry DOI: 10.7270/Q2PV6MR1 |
More data for this
Ligand-Target Pair | |
Sortase A (SrtA)
(Staphylococcus aureus) | BDBM50232478

(CHEMBL403233)Show SMILES O[C@@]12CC\C=C/CCCCN3CC[C@@H](C(=C1)c1nccc4c5ccccc5[nH]c14)[C@]1(C[C@@H]4\C=C/CCCCN4[C@@H]21)C3 |r,c:4,14,36| Show InChI InChI=1S/C36H44N4O/c41-36-18-10-4-1-2-5-11-20-39-22-17-30(35(25-39)23-26-13-7-3-6-12-21-40(26)34(35)36)29(24-36)32-33-28(16-19-37-32)27-14-8-9-15-31(27)38-33/h1,4,7-9,13-16,19,24,26,30,34,38,41H,2-3,5-6,10-12,17-18,20-23,25H2/b4-1-,13-7-/t26-,30-,34+,35-,36-/m0/s1 | PDB
MMDB
KEGG
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus ATCC 6538p SortA expressed in Escherichia coli using Dabcyl-QALPETGEE-Edans as substrate after 1 hr by fluorescen... |
J Nat Prod 80: 1575-1583 (2017)
Article DOI: 10.1021/acs.jnatprod.7b00121
BindingDB Entry DOI: 10.7270/Q2N87D8G |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50232478

(CHEMBL403233)Show SMILES O[C@@]12CC\C=C/CCCCN3CC[C@@H](C(=C1)c1nccc4c5ccccc5[nH]c14)[C@]1(C[C@@H]4\C=C/CCCCN4[C@@H]21)C3 |r,c:4,14,36| Show InChI InChI=1S/C36H44N4O/c41-36-18-10-4-1-2-5-11-20-39-22-17-30(35(25-39)23-26-13-7-3-6-12-21-40(26)34(35)36)29(24-36)32-33-28(16-19-37-32)27-14-8-9-15-31(27)38-33/h1,4,7-9,13-16,19,24,26,30,34,38,41H,2-3,5-6,10-12,17-18,20-23,25H2/b4-1-,13-7-/t26-,30-,34+,35-,36-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3-beta |
Bioorg Med Chem 16: 6702-6 (2008)
Article DOI: 10.1016/j.bmc.2008.05.079
BindingDB Entry DOI: 10.7270/Q2MP545W |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50232478

(CHEMBL403233)Show SMILES O[C@@]12CC\C=C/CCCCN3CC[C@@H](C(=C1)c1nccc4c5ccccc5[nH]c14)[C@]1(C[C@@H]4\C=C/CCCCN4[C@@H]21)C3 |r,c:4,14,36| Show InChI InChI=1S/C36H44N4O/c41-36-18-10-4-1-2-5-11-20-39-22-17-30(35(25-39)23-26-13-7-3-6-12-21-40(26)34(35)36)29(24-36)32-33-28(16-19-37-32)27-14-8-9-15-31(27)38-33/h1,4,7-9,13-16,19,24,26,30,34,38,41H,2-3,5-6,10-12,17-18,20-23,25H2/b4-1-,13-7-/t26-,30-,34+,35-,36-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Inhibition of GSK3-beta |
J Nat Prod 71: 1218-21 (2008)
Article DOI: 10.1021/np800163u
BindingDB Entry DOI: 10.7270/Q2GX4CGM |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50232478

(CHEMBL403233)Show SMILES O[C@@]12CC\C=C/CCCCN3CC[C@@H](C(=C1)c1nccc4c5ccccc5[nH]c14)[C@]1(C[C@@H]4\C=C/CCCCN4[C@@H]21)C3 |r,c:4,14,36| Show InChI InChI=1S/C36H44N4O/c41-36-18-10-4-1-2-5-11-20-39-22-17-30(35(25-39)23-26-13-7-3-6-12-21-40(26)34(35)36)29(24-36)32-33-28(16-19-37-32)27-14-8-9-15-31(27)38-33/h1,4,7-9,13-16,19,24,26,30,34,38,41H,2-3,5-6,10-12,17-18,20-23,25H2/b4-1-,13-7-/t26-,30-,34+,35-,36-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Inhibition of GSK3alpha |
J Nat Prod 71: 1218-21 (2008)
Article DOI: 10.1021/np800163u
BindingDB Entry DOI: 10.7270/Q2GX4CGM |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50232478

(CHEMBL403233)Show SMILES O[C@@]12CC\C=C/CCCCN3CC[C@@H](C(=C1)c1nccc4c5ccccc5[nH]c14)[C@]1(C[C@@H]4\C=C/CCCCN4[C@@H]21)C3 |r,c:4,14,36| Show InChI InChI=1S/C36H44N4O/c41-36-18-10-4-1-2-5-11-20-39-22-17-30(35(25-39)23-26-13-7-3-6-12-21-40(26)34(35)36)29(24-36)32-33-28(16-19-37-32)27-14-8-9-15-31(27)38-33/h1,4,7-9,13-16,19,24,26,30,34,38,41H,2-3,5-6,10-12,17-18,20-23,25H2/b4-1-,13-7-/t26-,30-,34+,35-,36-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Non-ATP competitive inhibition of GSK3beta |
Bioorg Med Chem Lett 22: 7232-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.043
BindingDB Entry DOI: 10.7270/Q2KD2034 |
More data for this
Ligand-Target Pair | |
Cyclin-Dependent Kinase 5 (CDK5)
(Homo sapiens (Human)) | BDBM50232478

(CHEMBL403233)Show SMILES O[C@@]12CC\C=C/CCCCN3CC[C@@H](C(=C1)c1nccc4c5ccccc5[nH]c14)[C@]1(C[C@@H]4\C=C/CCCCN4[C@@H]21)C3 |r,c:4,14,36| Show InChI InChI=1S/C36H44N4O/c41-36-18-10-4-1-2-5-11-20-39-22-17-30(35(25-39)23-26-13-7-3-6-12-21-40(26)34(35)36)29(24-36)32-33-28(16-19-37-32)27-14-8-9-15-31(27)38-33/h1,4,7-9,13-16,19,24,26,30,34,38,41H,2-3,5-6,10-12,17-18,20-23,25H2/b4-1-,13-7-/t26-,30-,34+,35-,36-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK5/p35 |
J Nat Prod 70: 1397-405 (2007)
Article DOI: 10.1021/np060092r
BindingDB Entry DOI: 10.7270/Q2R78G2K |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50232478

(CHEMBL403233)Show SMILES O[C@@]12CC\C=C/CCCCN3CC[C@@H](C(=C1)c1nccc4c5ccccc5[nH]c14)[C@]1(C[C@@H]4\C=C/CCCCN4[C@@H]21)C3 |r,c:4,14,36| Show InChI InChI=1S/C36H44N4O/c41-36-18-10-4-1-2-5-11-20-39-22-17-30(35(25-39)23-26-13-7-3-6-12-21-40(26)34(35)36)29(24-36)32-33-28(16-19-37-32)27-14-8-9-15-31(27)38-33/h1,4,7-9,13-16,19,24,26,30,34,38,41H,2-3,5-6,10-12,17-18,20-23,25H2/b4-1-,13-7-/t26-,30-,34+,35-,36-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Louisiana at Monroe
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GSK3-beta by ELISA |
Bioorg Med Chem 17: 6032-9 (2009)
Article DOI: 10.1016/j.bmc.2009.06.054
BindingDB Entry DOI: 10.7270/Q2N87BQG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data