 Found 63 hits for monomerid = 50235833
Found 63 hits for monomerid = 50235833 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50235833
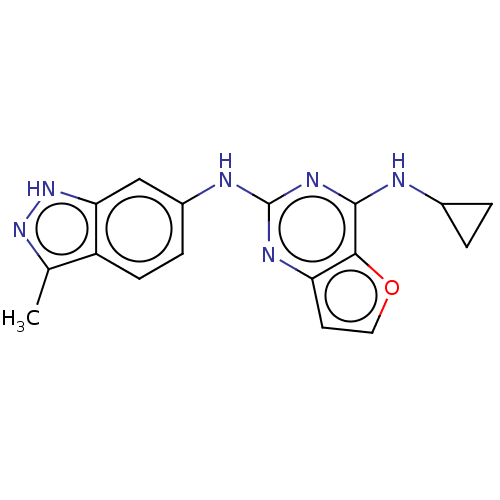
(CHEMBL4062803)Show InChI InChI=1S/C17H16N6O/c1-9-12-5-4-11(8-14(12)23-22-9)19-17-20-13-6-7-24-15(13)16(21-17)18-10-2-3-10/h4-8,10H,2-3H2,1H3,(H,22,23)(H2,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1(unknown origin) after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 2
(Homo sapiens (Human)) | BDBM50235833

(CHEMBL4062803)Show InChI InChI=1S/C17H16N6O/c1-9-12-5-4-11(8-14(12)23-22-9)19-17-20-13-6-7-24-15(13)16(21-17)18-10-2-3-10/h4-8,10H,2-3H2,1H3,(H,22,23)(H2,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of MEK2 (unknown origin) after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 2
(Homo sapiens (Human)) | BDBM50235833

(CHEMBL4062803)Show InChI InChI=1S/C17H16N6O/c1-9-12-5-4-11(8-14(12)23-22-9)19-17-20-13-6-7-24-15(13)16(21-17)18-10-2-3-10/h4-8,10H,2-3H2,1H3,(H,22,23)(H2,18,19,20,21) | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of MAP4K2 (unknown origin) after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50235833

(CHEMBL4062803)Show InChI InChI=1S/C17H16N6O/c1-9-12-5-4-11(8-14(12)23-22-9)19-17-20-13-6-7-24-15(13)16(21-17)18-10-2-3-10/h4-8,10H,2-3H2,1H3,(H,22,23)(H2,18,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of RET (unknown origin) after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50235833

(CHEMBL4062803)Show InChI InChI=1S/C17H16N6O/c1-9-12-5-4-11(8-14(12)23-22-9)19-17-20-13-6-7-24-15(13)16(21-17)18-10-2-3-10/h4-8,10H,2-3H2,1H3,(H,22,23)(H2,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of GSK3B (unknown origin) after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50235833

(CHEMBL4062803)Show InChI InChI=1S/C17H16N6O/c1-9-12-5-4-11(8-14(12)23-22-9)19-17-20-13-6-7-24-15(13)16(21-17)18-10-2-3-10/h4-8,10H,2-3H2,1H3,(H,22,23)(H2,18,19,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
In vitro antagonist activity at recombinant Metabotropic glutamate receptor 5 expressed in RGT cells. |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50235833

(CHEMBL4062803)Show InChI InChI=1S/C17H16N6O/c1-9-12-5-4-11(8-14(12)23-22-9)19-17-20-13-6-7-24-15(13)16(21-17)18-10-2-3-10/h4-8,10H,2-3H2,1H3,(H,22,23)(H2,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 (unknown origin) after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50235833

(CHEMBL4062803)Show InChI InChI=1S/C17H16N6O/c1-9-12-5-4-11(8-14(12)23-22-9)19-17-20-13-6-7-24-15(13)16(21-17)18-10-2-3-10/h4-8,10H,2-3H2,1H3,(H,22,23)(H2,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of AURKA (unknown origin) after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM50235833

(CHEMBL4062803)Show InChI InChI=1S/C17H16N6O/c1-9-12-5-4-11(8-14(12)23-22-9)19-17-20-13-6-7-24-15(13)16(21-17)18-10-2-3-10/h4-8,10H,2-3H2,1H3,(H,22,23)(H2,18,19,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of ACVR1 (unknown origin) after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 8
(Homo sapiens (Human)) | BDBM50235833

(CHEMBL4062803)Show InChI InChI=1S/C17H16N6O/c1-9-12-5-4-11(8-14(12)23-22-9)19-17-20-13-6-7-24-15(13)16(21-17)18-10-2-3-10/h4-8,10H,2-3H2,1H3,(H,22,23)(H2,18,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of CDK8 (unknown origin) after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50235833

(CHEMBL4062803)Show InChI InChI=1S/C17H16N6O/c1-9-12-5-4-11(8-14(12)23-22-9)19-17-20-13-6-7-24-15(13)16(21-17)18-10-2-3-10/h4-8,10H,2-3H2,1H3,(H,22,23)(H2,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 34 | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of Syk in human Ramos B cells assessed as decrease in intracellular calcium flux measured for 3.5 mins by calcium-5 dye based FLIPR assay |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
NT-3 growth factor receptor
(Homo sapiens (Human)) | BDBM50235833

(CHEMBL4062803)Show InChI InChI=1S/C17H16N6O/c1-9-12-5-4-11(8-14(12)23-22-9)19-17-20-13-6-7-24-15(13)16(21-17)18-10-2-3-10/h4-8,10H,2-3H2,1H3,(H,22,23)(H2,18,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Binding affinity to cloned human Dopamine receptor D4 expressed in CHO cells using [3H]spiperone |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50235833

(CHEMBL4062803)Show InChI InChI=1S/C17H16N6O/c1-9-12-5-4-11(8-14(12)23-22-9)19-17-20-13-6-7-24-15(13)16(21-17)18-10-2-3-10/h4-8,10H,2-3H2,1H3,(H,22,23)(H2,18,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibitory concentration against cyclin dependent kinase-2 (CDK2)/Cyclin E |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data