 Found 10 hits for monomerid = 50246060
Found 10 hits for monomerid = 50246060 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50246060
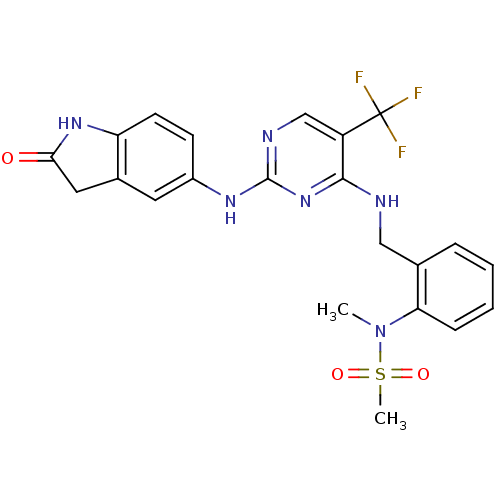
(CHEMBL472212 | CHEMBL541649 | N-methyl-N-(2-((2-(2...)Show SMILES CN(c1ccccc1CNc1nc(Nc2ccc3NC(=O)Cc3c2)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C22H21F3N6O3S/c1-31(35(2,33)34)18-6-4-3-5-13(18)11-26-20-16(22(23,24)25)12-27-21(30-20)28-15-7-8-17-14(9-15)10-19(32)29-17/h3-9,12H,10-11H2,1-2H3,(H,29,32)(H2,26,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged FAK (unknown origin) assessed as inhibition of poly-Glu-Tyr phosphorylation |
Bioorg Med Chem Lett 18: 6071-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.030
BindingDB Entry DOI: 10.7270/Q2C8295H |
More data for this
Ligand-Target Pair | |
Protein-tyrosine kinase 2-beta
(Homo sapiens (Human)) | BDBM50246060

(CHEMBL472212 | CHEMBL541649 | N-methyl-N-(2-((2-(2...)Show SMILES CN(c1ccccc1CNc1nc(Nc2ccc3NC(=O)Cc3c2)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C22H21F3N6O3S/c1-31(35(2,33)34)18-6-4-3-5-13(18)11-26-20-16(22(23,24)25)12-27-21(30-20)28-15-7-8-17-14(9-15)10-19(32)29-17/h3-9,12H,10-11H2,1-2H3,(H,29,32)(H2,26,27,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Pyk2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 18: 6071-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.030
BindingDB Entry DOI: 10.7270/Q2C8295H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50246060

(CHEMBL472212 | CHEMBL541649 | N-methyl-N-(2-((2-(2...)Show SMILES CN(c1ccccc1CNc1nc(Nc2ccc3NC(=O)Cc3c2)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C22H21F3N6O3S/c1-31(35(2,33)34)18-6-4-3-5-13(18)11-26-20-16(22(23,24)25)12-27-21(30-20)28-15-7-8-17-14(9-15)10-19(32)29-17/h3-9,12H,10-11H2,1-2H3,(H,29,32)(H2,26,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FAK |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Protein-tyrosine kinase 2-beta
(Homo sapiens (Human)) | BDBM50246060

(CHEMBL472212 | CHEMBL541649 | N-methyl-N-(2-((2-(2...)Show SMILES CN(c1ccccc1CNc1nc(Nc2ccc3NC(=O)Cc3c2)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C22H21F3N6O3S/c1-31(35(2,33)34)18-6-4-3-5-13(18)11-26-20-16(22(23,24)25)12-27-21(30-20)28-15-7-8-17-14(9-15)10-19(32)29-17/h3-9,12H,10-11H2,1-2H3,(H,29,32)(H2,26,27,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PYK2 |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50246060

(CHEMBL472212 | CHEMBL541649 | N-methyl-N-(2-((2-(2...)Show SMILES CN(c1ccccc1CNc1nc(Nc2ccc3NC(=O)Cc3c2)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C22H21F3N6O3S/c1-31(35(2,33)34)18-6-4-3-5-13(18)11-26-20-16(22(23,24)25)12-27-21(30-20)28-15-7-8-17-14(9-15)10-19(32)29-17/h3-9,12H,10-11H2,1-2H3,(H,29,32)(H2,26,27,28,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 445 | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human His6-TEV protease-tagged BRD4 bromodomain 1 expressed in bacteria by isothermal titration calorimetric method |
Nat Rev Drug Discov 16: 424-440 (2017)
Article DOI: 10.1038/nrd.2016.266
BindingDB Entry DOI: 10.7270/Q2125VNC |
More data for this
Ligand-Target Pair | |
Protein-tyrosine kinase 2-beta
(Homo sapiens (Human)) | BDBM50246060

(CHEMBL472212 | CHEMBL541649 | N-methyl-N-(2-((2-(2...)Show SMILES CN(c1ccccc1CNc1nc(Nc2ccc3NC(=O)Cc3c2)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C22H21F3N6O3S/c1-31(35(2,33)34)18-6-4-3-5-13(18)11-26-20-16(22(23,24)25)12-27-21(30-20)28-15-7-8-17-14(9-15)10-19(32)29-17/h3-9,12H,10-11H2,1-2H3,(H,29,32)(H2,26,27,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PYK2 by PYK2-LI-COR cellular assay |
Bioorg Med Chem Lett 19: 3253-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.093
BindingDB Entry DOI: 10.7270/Q2GH9J96 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50246060

(CHEMBL472212 | CHEMBL541649 | N-methyl-N-(2-((2-(2...)Show SMILES CN(c1ccccc1CNc1nc(Nc2ccc3NC(=O)Cc3c2)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C22H21F3N6O3S/c1-31(35(2,33)34)18-6-4-3-5-13(18)11-26-20-16(22(23,24)25)12-27-21(30-20)28-15-7-8-17-14(9-15)10-19(32)29-17/h3-9,12H,10-11H2,1-2H3,(H,29,32)(H2,26,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of purified activated FAK kinase domain (410-689) using ATP and Glu and Tyr random peptide polymer substrate by fluorescence polarization ... |
Bioorg Med Chem Lett 19: 3253-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.093
BindingDB Entry DOI: 10.7270/Q2GH9J96 |
More data for this
Ligand-Target Pair | |
Protein-tyrosine kinase 2-beta
(Homo sapiens (Human)) | BDBM50246060

(CHEMBL472212 | CHEMBL541649 | N-methyl-N-(2-((2-(2...)Show SMILES CN(c1ccccc1CNc1nc(Nc2ccc3NC(=O)Cc3c2)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C22H21F3N6O3S/c1-31(35(2,33)34)18-6-4-3-5-13(18)11-26-20-16(22(23,24)25)12-27-21(30-20)28-15-7-8-17-14(9-15)10-19(32)29-17/h3-9,12H,10-11H2,1-2H3,(H,29,32)(H2,26,27,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PYK2 |
Proc Natl Acad Sci USA 104: 10619-24 (2007)
Article DOI: 10.1073/pnas.0701421104
BindingDB Entry DOI: 10.7270/Q29887WR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein-tyrosine kinase 2-beta
(Homo sapiens (Human)) | BDBM50246060

(CHEMBL472212 | CHEMBL541649 | N-methyl-N-(2-((2-(2...)Show SMILES CN(c1ccccc1CNc1nc(Nc2ccc3NC(=O)Cc3c2)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C22H21F3N6O3S/c1-31(35(2,33)34)18-6-4-3-5-13(18)11-26-20-16(22(23,24)25)12-27-21(30-20)28-15-7-8-17-14(9-15)10-19(32)29-17/h3-9,12H,10-11H2,1-2H3,(H,29,32)(H2,26,27,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged thrombin cleavage site-fused human recombinant PYK2 catalytic domain (416 to 692 residues) expressed in baculovi... |
Nat Rev Drug Discov 16: 424-440 (2017)
Article DOI: 10.1038/nrd.2016.266
BindingDB Entry DOI: 10.7270/Q2125VNC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein-tyrosine kinase 2-beta
(Homo sapiens (Human)) | BDBM50246060

(CHEMBL472212 | CHEMBL541649 | N-methyl-N-(2-((2-(2...)Show SMILES CN(c1ccccc1CNc1nc(Nc2ccc3NC(=O)Cc3c2)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C22H21F3N6O3S/c1-31(35(2,33)34)18-6-4-3-5-13(18)11-26-20-16(22(23,24)25)12-27-21(30-20)28-15-7-8-17-14(9-15)10-19(32)29-17/h3-9,12H,10-11H2,1-2H3,(H,29,32)(H2,26,27,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PYK2 assessed as reduction in peptide substrate phosphoryltion by fluorimetric method |
Bioorg Med Chem Lett 19: 3253-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.093
BindingDB Entry DOI: 10.7270/Q2GH9J96 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data