

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50250677 CHEMBL4100845::US10961200, Compound 52
SMILES: NS(=O)(=O)c1ccc(Cc2c(nn(-c3nc(cs3)C(O)=O)c2C2CC2)-c2cccc(c2)-c2ccccc2)cc1
InChI Key: InChIKey=JXSFBKRZYZNXNV-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lactate dehydrogenase B (LDHB) (Homo sapiens (Human)) | BDBM50250677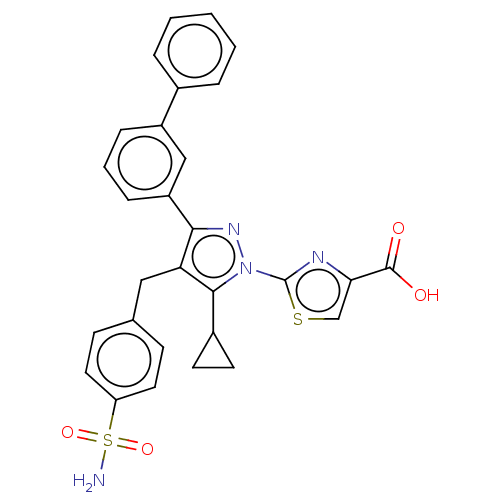 (CHEMBL4100845 | US10961200, Compound 52) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human erythrocytes LDHB using sodium pyruvate as substrate after 5 mins in presence of NAPDH by diaphorase/resazurin based fluorescence... | J Med Chem 60: 9184-9204 (2017) Article DOI: 10.1021/acs.jmedchem.7b00941 BindingDB Entry DOI: 10.7270/Q2DJ5J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50250677 (CHEMBL4100845 | US10961200, Compound 52) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Binding affinity to human His-tagged LDHA in presence of NADH by SPR assay | J Med Chem 60: 9184-9204 (2017) Article DOI: 10.1021/acs.jmedchem.7b00941 BindingDB Entry DOI: 10.7270/Q2DJ5J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50250677 (CHEMBL4100845 | US10961200, Compound 52) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of wild type IDH1 (unknown origin) using isocitrate as substrate preincubated for 30 mins followed by substrate addition in presence of NA... | J Med Chem 60: 9184-9204 (2017) Article DOI: 10.1021/acs.jmedchem.7b00941 BindingDB Entry DOI: 10.7270/Q2DJ5J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50250677 (CHEMBL4100845 | US10961200, Compound 52) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 983 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of LDH in human A673 cells assessed as reduction in lactate production preincubated for 2 hrs measured after 30 mins by high throughput fl... | J Med Chem 60: 9184-9204 (2017) Article DOI: 10.1021/acs.jmedchem.7b00941 BindingDB Entry DOI: 10.7270/Q2DJ5J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50250677 (CHEMBL4100845 | US10961200, Compound 52) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
THE UNITED STATES OF AMERICA, AS REPRESENTED BY THE SECRETARY, DEPARTMENT OF HEALTH AND HUMAN SERVICES; VANDERBILT UNIVERSITY; THE UAB RESEARCH FOUNDATION; THE TRUSTEES OF THE UNIVERSITY OF PENNSYLVANIA US Patent | Assay Description Test compounds were placed in a Greiner Bio-One (Monroe, N.C.) 1536-well black solid bottom assay plate. 200 millimolar (mM) Tris HCl, pH 7.4, 100 mi... | US Patent US10961200 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50250677 (CHEMBL4100845 | US10961200, Compound 52) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of LDH in human MIAPaCa2 cells assessed as reduction in lactate production preincubated for 2 hrs measured after 30 mins by high throughpu... | J Med Chem 60: 9184-9204 (2017) Article DOI: 10.1021/acs.jmedchem.7b00941 BindingDB Entry DOI: 10.7270/Q2DJ5J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50250677 (CHEMBL4100845 | US10961200, Compound 52) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human liver LDHA using sodium pyruvate as substrate after 5 mins in presence of NAPDH and in absence of EDTA by diaphorase/resazurin ba... | J Med Chem 60: 9184-9204 (2017) Article DOI: 10.1021/acs.jmedchem.7b00941 BindingDB Entry DOI: 10.7270/Q2DJ5J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50250677 (CHEMBL4100845 | US10961200, Compound 52) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 250 | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Stabilization of LDHA in human A673 cell lysate preincubated for 20 mins followed by incubation at 70 degreeC for 10 mins by cellular thermal shift a... | J Med Chem 60: 9184-9204 (2017) Article DOI: 10.1021/acs.jmedchem.7b00941 BindingDB Entry DOI: 10.7270/Q2DJ5J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50250677 (CHEMBL4100845 | US10961200, Compound 52) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human liver LDHA using sodium pyruvate as substrate after 5 mins in presence of NAPDH and EDTA by diaphorase/resazurin based fluorescen... | J Med Chem 60: 9184-9204 (2017) Article DOI: 10.1021/acs.jmedchem.7b00941 BindingDB Entry DOI: 10.7270/Q2DJ5J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||